Introduction
Milk is taken into account a whole feed and a building block of a spread of diets. Since, it’s a balanced supply of essential nutrients including protein, fat, minerals etc. Milk is wide consumed throughout the globe by all age teams, significantly infants, youngsters and senior individuals. Globally quite six billion individuals square measure the patron of milk and milk merchandise. Despite high nutritional values and tremendous usage, milk is according to be contaminated with a spread of adulterants, chemical and pharmaceutical compounds. The incidence of drug residues affects the standard of milk and milk merchandise and a better concentration than most permissible limits will manufacture important health hazards to the shoppers. The Food Safety and Standards Act, 2006, describes residues of a veterinary drug as “The parent compounds or their metabolites or each in any edible portion of any animal material and embody residues of associated impurities of the veterinary medication concerned”. The term food safety usually applied to food quality which will cause harmful effects in humankind and includes animal disease diseases and adverse effects made by xenobiotics. Recently, the presence of residues in milk and farm merchandise and their impact on public health is recognized as a matter of great concern. The country has an outsized population of milk-producing animals and also the use of veterinary medication for the treatment of sick animals is an integral a part of such intensive cultivation practices. The pharmaceutical merchandise employed in medical specialty have a very big selection that ranging from sex organ dips to the hormones. Therefore, it’s not unlikely that the drug residues seem within the milk and milk merchandise. so as to assure consumer’s safety and good-quality farm merchandise meant for export, milk ought to be often screened for drug residues.
Sources of Milk Contamination
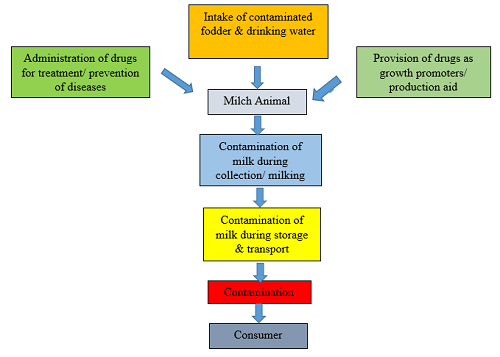
Contamination of milk starts right from the intake of contaminated pasture and beverage by the animal. Further, inappropriate use of veterinary medication in farm animals while not considering the withholding periods causes drug residues in milk. except for these, the contaminants can also be introduced throughout milk assortment, preservation, transport, process and packaging. Therefore, the milk can be the potential supply of drug residues and contaminants within the human diet. Most of the residues within the milk and milk merchandise square measure pharmaceutical medication like antimicrobials, anthelminthic agents, hormones etc. However, alternative compounds like pesticides, herbicides, detergents, disinfectants, mycotoxins, nitrates, nitrites and serious metals also are detected as mentioned below in Table 1.
Table 1: Common chemical and drug residues appearing in the milk
| Class | Drugs/ chemicals |
| Antibiotics | Penicillin, Oxytetracycline, Streptomycin, Neomycin, Tetracycline |
| Antihelminthics | Closantel, Ivermectin, Levamisole, Albendazole |
| Pesticides | Organochlorines, Organophosphates, DDT, HCHs |
| Hormones | Bovine somatotropin, Progesterone, Testosterone |
| Mycotoxins | Aflatoxin M1, Aflatoxin B1 |
| Nitrates and Nitrites | Nitrate fertilizers, Rodenticides |
| Heavy Metals | Lead (Pb), Cadmium (Cd) |

These medications are continually excreted into the milk during treatment and after a particular period of time (withdrawal period) of discontinuation of pharmacological therapy. In theory, all of these routes could result in residues in the milk, but intramammary infusions induce a higher prevalence of drug residues. Furthermore, improper milking or milk collection, insufficient cleaning, poor hygienic conditions, and other inappropriate management practises can contribute to drug and chemical contamination of milk.
Mode of action
They show some selectivity or varied toxicity to a range of bacterial cells under different conditions of application.
(1) On the cell wall to change its permeability or to cause lysis by increasing osmotic fragility
(2) On one or more enzymatic processes affecting metabolism in absorption, respiration, energy utilisation, synthesis of genetic, structural, or metabolic components, detoxication reactions
(3) On reproductive processes or chromosomal integrity
(4) On excretion
(5) On sensitivity mechanisms by increasing or decreasing them
(6) On integrity of defense systems
(7) On control of homeostasis in any of the other processes considered.
Impacts on Public Health
Even at higher concentrations, the majority of regularly used medications are relatively safe, although a handful of them can have substantial health consequences. Similarly, residual amounts of pharmaceuticals taken through animal origin food are not always hazardous unless they reach a specified level, i.e. MRL. Infants and growing children are especially vulnerable since they consume a substantial amount of milk and milk products based on their body weight. Some pollutants, such as penicillins, tetracyclines, chloramphenicol, streptomycin, and oxytetracycline, are not inactivated by pasteurisation, and even drying is ineffective at detoxifying penicillin residues in milk.
Hypersensitive Reaction
There are two forms of drug-mediated hypersensitivity reactions: IgE-mediated and non-IgE-mediated. IgE-mediated reactions include urticaria, anaphylaxis, bronchospasm, and angioedema, whereas non-IgE-mediated reactions include haemolytic anaemia, thrombocytopenia, acute interstitial nephritis, serum sickness, and vasculitis.
Drug Resistance
Antibiotic usage in livestock husbandry has been linked to the development of antimicrobial drug resistance (AMR) via the farm-to-fork food chain. Long-term usage of antibiotics at subtherapeutic doses as growth promoters is of particular concern. Antimicrobial resistance develops as a result of long-term low-dose antibiotic use via contaminated milk and milk products. The consumer’s normal flora and pathogenic bacteria are unnecessarily exposed to antibiotic residue at sub-therapeutic concentrations, resulting in the development of multi-drug resistance bacterial species such as Salmonella, Staphylococcus, Campylobacter, E. coli, and many more.
Teratogenicity
Teratogenicity refers to a drug’s or chemical’s ability to cause severe and toxic effects on a growing embryo or foetus during a vital stage of pregnancy. Some medications have been linked to developmental toxicity, embryotoxicity, or teratogenic consequences when used during pregnancy. Teratogenic medications include chemotherapeutic agents (thalidomide), hormones (diethylstilbestrol, misoprostol), and other drugs such as ACE inhibitors, cyclophosphamide, methimazole, and others.
Mutagenicity
Mutagenicity refers to a chemical or drug’s ability to cause changes in the DNA molecule or the genetic component of a cell in an organism. Some medications have the potential to cause DNA mutations or chromosomal damage, resulting in human infertility. Metronidazole, a nitroimidazole derivative, has been shown to be mutagenic and genotoxic. In human peripheral blood lymphocytes and hepatocytes, it caused DNA strand breakage and fragmentation, respectively.
Disruption of Normal Gut Flora
By competing with dangerous bacteria, normal gut flora functions as a barrier for them, preventing disease from occurring. Antibiotic residues in milk, such as nitroimidazole, metronidazole, tetracyclines, penicillins, streptomycin, and others, disrupt the normal gut flora, resulting in gastrointestinal disorders. Disruption of the natural bacterial flora of the gut can cause gastro-intestinal problems and allow pathogens to penetrate and multiply in the host. Pseudomembranous colitis, Clostridium difficile-associated diarrhoea, and other potentially fatal diseases can arise in severe cases.
Techniques for Detection of Drug Residues in Milk
There are several techniques available for the determination of different classes of drug residues in milk and dairy products as listed below-
- Bioassays
- Receptor assay
- Microbiological assays
- Enzymatic assays
- Enzyme-linked Immunosorbent Assay (ELISA)
- Thin Layer Chromatography (TLC)
- Paper chromatography
- Gas Chromatography-Mass Spectrometry (GC-MS)
Guidelines to Minimize Drug Residues in Milk and Milk Products
In practise, avoiding or completely eliminating chemical and drug residues from milk and milk products is difficult; however, the implementation of specific safety procedures and guidelines may aid in reducing residues to non-toxic levels. The rules are summarised as follows:
- Compliance with regulatory requirements on dairy farms and in the dairy industry.
- Educating farmers and animal keepers on the need of producing clean, contaminant-free milk.
- Animal farms and dairy units must follow sanitary and proper management standards.
- Carefully planned drug usage programme, including sensible antibiotic use, avoidance of pharmaceuticals not intended for dairy cows, and adherence to label recommendations.
- Withholding milk from animals on medicine for a set amount of time (withdrawal period), as prescribed by the authority or manufacturer. The best single method to eliminate the presence of detectable antibiotics in milk, simply stated, is to keep the milk from treated cows out of the central milk supply. With some exceptions, 72 hr. post-treatment is generally long enough.
- Promoting the use of risk-free alternative medicine and ethno-veterinary methods.
- In the livestock and dairy industries, the adoption of programmes such as Hazard Analysis Critical Control Point (HACCP)
Table 2: Acceptable Daily Intake (ADI) and Maximum Residual Limits (MPL) of some chemicals and veterinary drugs, in cow milk (Codex Alimentarius, 2011)
| Category | Drugs | ADI (μg/kg bw) | MRL (µg/kg) |
| Antimicrobial Agents | Amoxicillin
Gentamicin Tetracycline Oxytetracycline |
2
0-20 0-30 0-30 |
4
100 100 100 |
| Antihelminthics | Ivermectin
Albendazole Fenbendazole |
0-10
0-50 0-7 |
10
100 100 |
| Antiprotozoal drugs | Imidocarb
Diminazene |
0-10
0-100 |
50
150 |
| Insecticides | Deltamethrin
Cypermethrin |
0-10
0-20 |
30
100 |
| Hormones | Estradiol-17β
Bovine somatotropin |
0-0.05
NS |
NS
NS |
| Heavy metals | Lead | NS | 20 |
| Mycotoxins | Aflatoxin M1 | NS | 0.5 |
Conclusion
While milk is a nutritious, healthy, and extensively consumed food, it can contain a range of dangerous medication residues. Along with the significant increase in the dairy industry in terms of adapting modern technology in processing and production, there is an urgent need to monitor milk and milk product quality in order to assure public safety. Because dairy products are commonly ingested by newborns, children, and many people worldwide on a daily basis, pollutants such as drug residues in milk have substantial public health implications. It is extremely difficult to totally prevent or eradicate chemical and medication residues in milk and milk products. However, by implementing correct food safety precautions, drug residues can be reduced to a safe level. As a result of the collaboration and involvement of farmers, doctors, manufacturers, researchers, consumers, and legislative and other food safety authorities, the overall public health impact of drug residues in milk and milk products can be reduced.





Be the first to comment