Introduction
Foot and mouth disease (FMD) is a highly contagious, severe viral disease that has a large economic impact on animals. Cattle, swine, sheep, goats, and other cloven-hoofed ruminants are all affected. It has a significant impact on livestock output while also affecting regional and worldwide animal and animal product trade. Despite the fact that FMD has a low fatality rate of less than 5%, it is nonetheless regarded the most important disease affecting farm animals because it causes significant losses in livestock productivity and commerce Although FMDV seldom causes death in adults, it can cause extensive myocardial damage in young animals, resulting in high fatality rates. The disease is thought to be present in 77 percent of the world’s cattle population, with the majority of cases occurring in Africa, the Middle East, and Asia, as well as a small region of South America (OIE). In the United Kingdom, the 2001 outbreak resulted in losses of more than 8 billion pounds (about $AUD 19 billion).
FMD is caused by etiological agent, foot-and-mouth disease virus (FMDV), that belongs to the genus Aphthovirus, in the family Picornaviridae. The virus comes in seven serologically and genetically distinct types: O, A, C, Asia1, SAT1, SAT2, and SAT3, however each serotype has a significant number of subtypes. FMDV is a single-stranded (ss) positive sense RNA virus with a sedimentation coefficient of 146S and a genomic size of around 8.5 Kb.
In India, foot and mouth diseases (FMD) is still the primary cause of cattle losses. Occasionally, outbreaks are reported throughout the year. Inadequate surveillance and diagnostic facilities, as well as insufficient control measures, are important obstacles to the country’s control of this disease. Aside from direct losses to the cattle industry, severe trade restrictions have an indirect impact that may be greater than direct losses. In order to effectively control the disease, outbreaks must be identified early on, and persistent infections must be identified to prevent future transmission. These can be attained if immunisation is routine and successful, and diagnostic methods are specific and sensitive while also being quick.
Epidemiology of the Disease
The epidemiology of FMD is complicated, and it is influenced by a variety of viral, host, and environmental factors, including virus virulence (the severity of lesions, the amount, and duration of virus release), particle stability in various microenvironments, and the likelihood of long-term persistence. FMDV multiplication and distribution are further influenced by the host species, nutritional and immunological state, population density, animal movements, and encounters between domestic and wild host species as well as animals capable of mechanical transmission.
FMD is a highly transmissible disease can be spread mechanically through contaminated animal products, non-susceptible animals, agricultural instruments, people, and airborne transmission particularly by milk tankers or animal transport vehicles, and virus transmission by the wind (Woodbury 1995). The transmission of FMDV from one animal to another by aerosol is one of the most common ways for the disease to spread (Alexandersen et al. 2003). The multiplication of FMDV is influenced by the host species, nutritional and immunological state, population density, animal movements, and interactions between domestic and wild host species as well as animals capable of mechanical transmission. When adequate atmospheric conditions prevail, the environment can act as a geographical barrier to virus spread or, conversely, might enhance virus transmission.
Diagnosis of FMD
The diagnosis of FMD depends particularly on typical clinical indicators, epidemiology, pathological abnormalities, and specific identification of the viral antigen or specific antibodies in suspected samples utilizing various serological and molecular techniques are all used to make a diagnosis of viral disease. In FMD-endemic areas, accurate FMDV infection detection is essential for control and eradication operations, as well as a support measure for the FMD-free approach
Clinical indications and macroscopic lesions on the tongue and feet, with or without a history of interaction between the herd and the sick animal, or a report of FMD outbreak in the surrounding area, lead to the diagnosis of FMD. Clinical signs in a highly vulnerable animal are pathognomic; however, clinical indications in an endemic location may be modest or confused due to partial natural immunity or vaccinal immunity.
Clinical examination, laboratory analysis (golden standard) via viral isolation (VI) in sensitive cell cultures, and numerous diagnostic procedures such as antigen detection enzyme linked immunosorbent assay (ag ELISA) and lateral flow assay (LFA) are used to diagnose disease. RT-PCR (Reverse transcription polymerase chain reaction) and reverse transcription–loop-mediated isothermal amplification (RT-LAMP) are two molecular detection approaches.
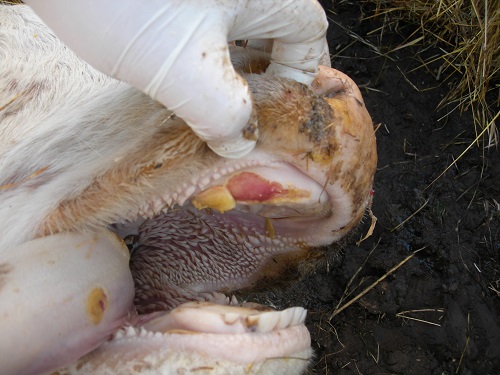
Clinical Signs
Fever usually appears 24 to 48 hours before indications develop in clinical disease the incubation time ranges from 2 to 14 days, depending on the infecting dose, virus strain, and susceptibility of the individual host. Animals with a high fever should be carefully inspected for lesions on the mouth, feet, or teat, as well as concurrent saliva dribbling and lameness with vesicles and/or erosions. Epithelial flaps and erosions can be noticed on the gingiva, dental pad, and hard palate, as well as more strikingly on the dorsum of the tongue, upon closer inspection of the buccal cavity. These lesions were previously entire aphthae or blisters. Acutely infected animals salivate abundantly and develop a mucoid nasal discharge that eventually becomes mucopurulent and coats the muzzle. High fever can cause abortion in pregnant animals (FMDV does not reach the placenta). Severe cardiac necrosis causes death in young animals. Laboratory testing can be used to distinguish FMD from other disorders such as swine vesicular disease, vesicular stomatitis, and vesicular exanthema of the mouth.
Livestock production losses might be significant. Animals that produce a lot of milk will not be able to recoup their prior yields; swine and cattle weight gain will also be impaired. In cases of FMD, mortality is uncommon unless young animals are involved. There is substantial cardiac involvement in these cases, which is characterized by myocardial necrosis, which may be seen macroscopically as whisks of blanched patches on the epicardium that extend into the endocardium. whisks of blanched patches on the epicardium that extend into the endocardium. Lambs, youngsters, piglets, and calves can have a case-fatality rate of more than 60%. Perhaps the previously observed production losses are the result of modest cardiac insufficiencies in mature animals who have recovered from acute illness episodes.
Serological Methods
The benefit of serological procedures is to detect viral antigens or antibodies in serum as well as in other body fluids. Anti-FMDV antibodies in serum samples is a confirmatory method for FMD diagnosis. The utility of serological tests are used for the purpose to certify individual animals before import or export (i.e. for trade), confirmation of suspected FMD cases, prove the absence of infection, and to demonstrate efficacy of vaccine.
The various tests that are used include Virus neutralization test (VNT), Complement fixation test (CFT), ELISA. Tissue is processed for virus isolation and antigen-capture ELISA; analysis is initiated using many accessible polymerase chain reaction (PCR) techniques; and, if tissue is sufficient, a complement fixation (CF) test is performed The first results on the submitted epithelium could be available in as little as 5 hours, but this is highly dependent on the sample quality and quantity. If the virus isolation is positive, it will be visible within 5 to 18 hours of seeding the sample over susceptible cells, but this finding will require confirmation of the isolated virus using additional techniques (again, processed by ELISA, PCR, or CF) and morphology viewing with an electron microscope.
Molecular-based approaches identify the presence of viral nucleic acids by their amplification and have a higher sensitivity than serological methods. For FMDV serotyping, primers have also been created. Because FMDV is an RNA virus, RT is essential before the specific viral nucleic acid can be amplified. RT-PCR and RT-LAMP are the most often utilised techniques. A 91-bp nucleotide fragment within the 3D coding region was detected using this method. This approach was found to be sensitive enough to detect 0.1 TCID50 of cell culture FMDV, according to the findings.
Vaccination
In the mid-1930s, formalin was used as an inactivant in the development of the first successful FMD vaccine for animals. In the 1950s and 1960s, there was a lot of interest in virus attenuation research, but the persistent existence of residual pathogenicity for some susceptible species in numerous laboratories around the world, often with significant consequences, made it an inefficient immunogen. Water-based (aluminium hydroxide–saponin), single-oil, or double-oil emulsion vaccinations are available. Vaccination is frequently used in combination with particular programmes to control or eradicate illness, hence vaccines are rarely available on the open market. If oil-based adjuvants are used in endemic areas, cattle vaccination could be given twice in the first year of life and once a year after that. Most regions, however, vaccinate all animals twice a year, regardless of age, to ensure high herd immunity and compliance.
“Vaccine banks” are common in FMD-free countries, where vaccine antigen concentrates are stored under liquid nitrogen and formulated into finished products in the event of an emergency. Such is the case in the United States, which, along with Mexico and Canada, shares a vaccine antigen bank (the North American Foot and Mouth Disease Vaccine Bank, located off the coast of New York) that contains numerous antigen concentrates for several major subtypes and would be activated immediately if FMD was detected in the region.
Conclusions
The foot-and-mouth disease virus (FMDV) is known to cause large-scale outbreaks. Many studies have been conducted to develop and validate diagnostic diagnostics for FMD. FMD Traditional methods for detecting FMDV infection are either not very specific or lack a high level of sensitivity, which should not be neglected when screening a herd. With the urgent need for new diagnostic tests to detect viral infection, researchers should focus their efforts on developing simple, quick, noninvasive assays that can be done without the use of expensive laboratory equipment.
References
- Grubman, M.J. and Baxt, B. (2004). Foot-and-mouth disease. Clin. Microbiol. Rev. 17 (2): 465–493.
- https://www.oie.int/en/disease/foot-and-mouth-disease/
- Knowles, N. J., Samuel, A. R., Davies, P. R., Kitching, R. P., & Donaldson, A. I. (2001). Outbreak of foot-and-mouth disease virus serotype O in the UK caused by a pandemic strain. The Veterinary Record, 148(9), 258-259.
- Longjam, N., Deb, R., Sarmah, A. K., Tayo, T., Awachat, V. B., & Saxena, V. K. (2011). A brief review on diagnosis of foot-and-mouth disease of livestock: conventional to molecular tools. Veterinary medicine international, 2011.
- Lubroth, J. (2002). Foot-and-mouth disease: a review for the practitioner. Veterinary Clinics: Food Animal Practice, 18(3), 475-499.
- Marquardt, O., Straub, O. C., Ahl, R., & Haas, B. (1995). Detection of foot-and-mouth disease virus in nasal swabs of asymptomatic cattle by RT-PCR within 24 hours. Journal of virological methods, 53(2-3), 255-261.
- Reid, S. M., Ferris, N. P., Hutchings, G. H., Zhang, Z., Belsham, G. J., & Alexandersen, S. (2002). Detection of all seven serotypes of foot-and-mouth disease virus by real-time, fluorogenic reverse transcription polymerase chain reaction assay. Journal of virological methods, 105(1), 67-80
- Rémond, M., Kaiser, C., & Lebreton, F. (2002). Diagnosis and screening of foot-and-mouth disease. Comparative immunology, microbiology and infectious diseases, 25(5-6), 309-320.






Be the first to comment