Introduction
In India, due to the ever-growing human population, there is an increased demand for food supplies. Milk is an important part of the human diet, a product of animal origin for day-to-day consumption. Ruminants being the major contributor of milk, they should be fed with a balanced ration for milk production. Rumen microorganisms play a prominent role in the digestion of ligno-cellulosic feedstuffs. The rumen is the major fermentation-vat that converts the components of the feed into useful (VFA and microbial protein) and unwanted (methane and carbon dioxide) end-products (Patra et al., 2012). In tropics, low-quality roughages, crop-residues, etc. containing a high level of ligno-celluloses, poor fermentable carbohydrate, and good quality protein are given for ruminants. Methods aiming to reform rumen manipulation are the key to enhance ruminant production and then optimizing utilization of feedstuffs.

In the Indian context, the cost of feeds, their quality, and their environmental effects are some of the major challenges faced. Hence, it is the need of the hour to look after modern and recent advancements made in the rumen manipulation techniques that can be utilized for boosting livestock production. In this article, we discuss the various objectives of rumen manipulation, its methods, and general effects
Rumen Manipulation
Rumen manipulation is expected to improve helpful processes while it reduces, removes, and changes the processes, which are dangerous to the host (Adesogan, 2009). It involves increased production in ruminants (viz. milk, wool, and meat production). This helps to optimize the efficiency of feedstuffs utilization.
Main objectives of Rumen Manipulation
- Enhance fibrolytic activity– Aids in the breakdown of lignocellulosic bonds
- To increase Microbial protein synthesis (MBP)
- Reduction in proteolysis- Reduction in degradation of protein and decrease in deamination of amino acids
- Reduction in methanogenesis- The provision of an alternate hydrogen sink
- Prevention of acidosis
- Shifting acetate to propionate production
- Novel microbes
- Plant toxins’ metabolism
- Helpful Secondary metabolites’ synthesis
(Santra and Karim, 2003; Patra et al., 2012)
Methods of Rumen Manipulation
- Genetic manipulation
- Non-genetic manipulation (Dietary manipulations)
A- Genetic Manipulation
Genetically engineered rumen microbes are developed. Gene transfer/manipulation techniques are used to increase the productivity of animals (Choudhury et al., 2015). Proper application of molecular techniques has tremendous potential to accomplish the goals of rumen handling.
Objectives of Genetic Manipulation
- To modify the distribution of fermentation products produced within the rumen to improve fermentation
- To produce metabolites (e.g., hormones) that can improve the metabolic efficiency of the animal
- To introduce improved or novel pathways for degradation of feedstuffs
- To suppress the growth and metabolic activities of undesirable organisms
(Santra and Karim, 2003)
Approaches of Genetic Manipulation
- Introduce new species or strains of micro-organisms into the gut
(Stewart et al., 1988)
- Genetic modification of micro-organisms already present in the rumen
(Chang, 1996)
- Common shuttle vector used- Escherichia coli
- Genes which have been cloned in Escherichia coli are endoglucanase, xylanase, β-glucosidase, amylase, glutamine synthetase
- Donor source of Bacteroides fibrisolvens, Ruminococcus flavefaciens, Fibrobacter succinogenes, Neocallimastix frontalis, Streptococcus bovis
Constraints of Genetic Manipulation
- Physico-chemical environment of the rumen is not favorable for most non-rumen microbes
- Low competing capability
- Adaptation to and tolerance of the chemical and physical environment in the rumen
- Competition with indigenous microbes for substrates that are used for growth
- Existing regulation about the release of genetically modified microbes in the atmosphere
Non-Genetic Rumen Manipulation
- Feed Additives
- Defaunation
- Plant Extracts
Feed Additives
Feed additives are compounds added to the diet to, improve performance, reduce the risk of metabolic disorders, improve dietary nutrient utilization and decrease the detrimental effects of diets on the environment.
Feed additives classified into
- Microbial Feed Additives (Probiotics)
- Non-Microbial Feed Additives
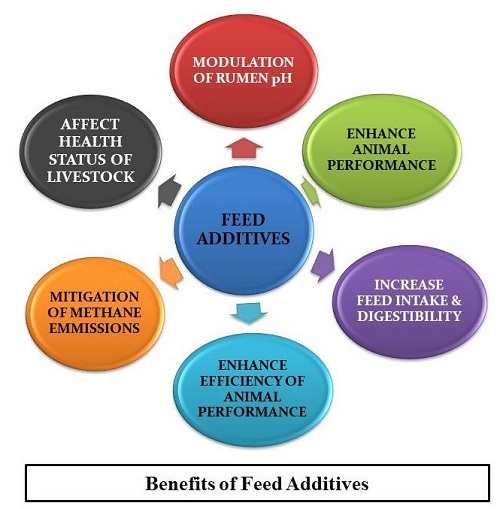
Characteristics of Feed Additives
- Ruminal pH modulation and decreased lactate accumulation
- Reduce the risk of development of diarrhea in neonates and ruminal acidosis or bloat in older livestock
- Increase the ruminal energy utilization efficiency
- Hasten rumen development in neonatal ruminants
- Increase ruminal organic matter & fiber digestibility
- Enhance the level and efficiency of animal performance
- Ameliorate the efficiency of ruminal nitrogen utilization
- Approved by legislative authorities
- Be cost-effective
(Bodas et al., 2012; Elghandour et al., 2015; Haque et al., 2018)
1. Probiotics
A live microbial feed supplement that has positive effects on the intestinal microbial balance of host animals (Fuller, 1989). These are Direct fed microbes (DFM) (FDA, 1995). FDA defines DFM as a source of live naturally occurring microorganisms and this includes bacteria and fungi/yeast (Miles and Bootwalla, 1991).
The commonly studied microorganisms used as probiotics in the ruminants feed include bacterial species belonging to the genera Bacillus, Enterococcus, Streptococcus, Bifidobacterium, Propionibacterium, Lactobacillus, and Prevotella bryantii, and yeast products containing Saccharomyces and Aspergillus (Adesogan, 2009). The most prominently used probiotic for commercial purposes in ruminants is Saccharomyces cerevisiae (Live yeast) (Elghandour et al., 2015).
Probiotics increase the milk yield, improves production performance, increases the growth rate in calves, develop immunity, dietary intake enhances, and reduces the emissions of methane gas that helps to reduce global warming (Elghandour et al., 2015; Haque, 2018).

2. Ionophores
Ionophores are organic compounds mainly obtained from Streptomyces species. They help to facilitate the transportation of ions selectively across the outer cell membrane. E.g. Monensin (widely used ionophore as `Rumensin’), Lasalocid, Tetronasin, Nigericin, Salinomycin, Lysocellin, Narasin, Laidlomycin and Valynomycin. Major countries of the world viz. Australia, USA, Argentina, South Africa, and New Zealand have permitted the use of ionophores in diets of the ruminants (Rusell et al., 2002).
Effect of Ionophores
Ionophores favor propionate production. The associated decrease in the production of methane that conserves energy in the ruminants. Inhibit the growth of gram-positive bacteria in the rumen. They cause a decrease in hydrogen and formate, a precursor of methane (Guan et al., 2006; Haque, 2018).
3. Organic Acids
Commonly used organic acids are Fumarate, Malate, and Aspartate. They stimulate cellulolytic bacteria and propionate production in the rumen. Organic acids act as an H2 sink, hence reducing the amount of methane (CH4) (Wallace, 2004; Castillo et al., 2004). Malate showed responses similar to those of ionophores (i.e., increased propionate, decreased methane, and decreased lactate) (McAllister and Newbold, 2008).
The combination of organic acids (Malate) and Monensin is much more effective at reducing lactate concentrations and pH in mixed ruminal microorganism fermentation than the addition of organic acid or monensin alone (McAllister and Newbold, 2008).
4. Essential Oils
Essential oils are secondary plant metabolites, aromatic lipophilic compounds and volatile components. They have extremely potent antimicrobial properties that prevent the growth and survival of the majority of microorganisms in the rumen (Benchaar et al., 2008). In particular, they help to modulate cellular targets by interacting with ion gradients, phosphorylation, ATP production, and protein translocation (Benchaar et al., 2008). Like monensin, selectively inhibit gram-positive bacteria (Trombetta et al., 2005).
5. Exogenous Fibrolytic Enzymes
Exogenous fibrolytic enzymes are the commercial mixtures of cellulase and hemicellulase enzymes. They have varying activities of exoglucanase, endoglucanase, β-glucosidase, xylanase, protease; activities have ensured at hydrolyzing plant cell walls. Mainly improves digestibility of fibers and enhances animal productivity. Further, lower the acetate: propionate ratio in the rumen and ultimately reducing CH4 production (Eun et al., 2007; Haque, 2018).
6. Fat Supplementation
Fat supplementation increases the dietary energy content. Among fatty acids, the medium-chain C8:C14 from coconut or palm oil is the most effective in CH4 mitigation (Costa et al., 2017). Furthermore, fats are not metabolized in the rumen and therefore do not contribute to methanogenesis (Giger-Reverdin et al., 2003).
Defaunation
The process of making the rumen of animals free of rumen protozoa is called defaunation and the animal is called defaunated animal.
Benefits of Defaunation
- Reduces the ruminal methane (CH4) production
- Increases protein outflow in the intestine,
- Results in improved growth and feed conversion efficiency of the animals
- Increases the number of amylolytic bacteria
Methods of Defaunation
Ⅰ. Isolation of newborn animals
- Separation of newborn animals from their dams after birth
- Preventing them from any contact with the adult ruminant animals
- Separated 2 to 3 days after birth
Ⅱ. Chemical treatment
- Copper sulphate
- Manoxol
- Sodium lauryl sulphate
(Santra and Karim, 2003)
Ⅲ. Dietary Manipulation
On supplementation of high-energy feed like maize, barley (cereal grains) to the starved animals (up to 24 hours) results in increased acid production in the rumen. Due to this, the rumen pH declines below 5.0 leading to Ruminal acidosis. Ciliate protozoa are very much sensitive to rumen pH below 5.0 and lead to their elimination. Hence, an animal is defaunated (Pen et al., 2006).
Plant Extracts
The antimicrobial activity of plant extracts – Secondary plant metabolites (saponins, tannins, Anthraquinone, and Sinigrin) is prominent. Certain plants exhibit anti-methanogenic activity. Such plants are Equisetum arvense, Rheum palmatum, Salvia officinalis, Lotus corniculatus, Sapindus saponaria, Uncaria gambir and Yucca schidigera. (Mueller-Harvey, 2006; Goel and Makkar, 2012). Major commercial source of saponins- Yucca schidiger
1. Condensed Tannins
The cultivation of forages with higher tannin levels, such as clover and other legumes, along with vetch, Sulla, chicory, and trefoil promotes mitigation of CH4 (up to 55%) (Bodas et al., 2012). Condensed tannins can bind protein by hydrogen bonding at near-neutral pH (pH 6.0 to 7.0) in the rumen to form CT-protein complexes. Afterwards, they dissociate and release bound protein at pH less than 3.5 on entering the abomasum and small intestine (Mueller-Harvey, 2006). VFA production increases and results in the shift from acetate to propionate production (Patra and Saxena et al., 2009). Feeds supplemented with tannins or tannin-rich forages, which bind proteases and thus prevent proteolysis (Zhang et al., 2019).
Limitations
Impede forage digestibility and animal productivity when fed at a higher concentration (Beauchemin et al., 2008).
2. Saponins
Saponins are naturally occurring surface-active glycosides that are found in a wide variety of cultivated and wild plant species (Tamminga et al., 2007). They are antiprotozoal at lower concentrations (Bodas et al., 2012). Higher concentrations can suppress Methanogens up to 50% (Patra and Saxena, 2009; Haque, 2018).
Conclusions
Rumen should be manipulated essentially by altering the composition of rumen microflora, feeding local plants or tree leaves to defaunate the animals for improving its productivity. Proper application of molecular techniques has tremendous potential to accomplish the goals of efficient genetic manipulation of rumen microorganisms. In India, further emphasis can be provided for the manipulation of the rumen to improve the activity of cellulolytic bacteria, which helps in the proper utilization of low-quality roughages.
Reference
- Adesogan T. A. (2009). Using Dietary Additives to Manipulate Rumen Fermentation and Improve Nutrient Utilization and Animal Performance. University of Florida
- Beauchemin KA, Kreuzer M, O’Mara F, McAllister TA. Nutritional management for enteric methane abatement: a review. Aust J Exp Agric. 2008;48:21–7.
- Benchaar C, Calsamiglia S, Chaves AV, Fraser GR, Colombatto D, McAllister TA, Beauchemin KA. A review of plant-derived essential oils in ruminant nutrition and production. Anim Feed Sci Technol. 2008;145:209–28.
- Bodas R, Prieto N, García-González R, Andrés S, Giráldez FJ, López S. Manipulation of rumen fermentation and methane production with plant secondary metabolites. Anim Feed Sci Technol. 2012;176:78–93.
- Castillo C, Benedito JL, Mendez J, Pereira V, Lopez-Alonso M, Miranda M, Hernandez J. Organic acids as a substitute for monensin in diets for beef cattle. Anim Feed Sci Technol. 2004;115:101–16.
- Chang, H. 1996. Genetic engineering to enhance microbial interference and related therapeutic applications. Nature Biotecnol. 14:423-431.
- Choudhury P.K., Salem A.Z.M., Jena R., Kumar S., Singh R., Puniya A.K. (2015) Rumen Microbiology: An Overview. In: Puniya A., Singh R., Kamra D. (eds) Rumen Microbiology: From Evolution to Revolution. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2401-3_1
- Costa M, Alves SP, Cabo A, et al. Modulation of in vitro rumen biohydrogenation by Cistus ladanifer tannins compared with other tannin sources. J Sci Food Agric 2017;97(2):629–35.
- Elghandour M.M., Salem A.Z., Castañeda J.M., Camacho L.M., Kholif A.E., Chagoyán J.V. (2015). Direct-fed microbes: A tool for improving the utilization of low quality roughages in ruminants. J. Integr Agric., 14: 526-533.
- Eun JS, Beauchemin KA. Assessment of the efficacy of varying experimental exogenous fibrolytic enzymes using in vitro fermentation characteristics. Anim Feed Sci Technol. 2007;132:298–315.
- FDA (1995) Direct-fed microbial products. Compliancy Policy Guide, Silver Spring
- Fuller R (1989) A Review: Probiotics in man and animals. J Appl Bacteriol 66: 365-378.
- Giger-Reverdin S, Morand-Fehr P, Tran G. Literature survey of the influence of dietary fat composition on methane production in dairy cattle. Livest Prod Sci. 2003;82:73–9.
- Goel G, Makkar HP. Methane mitigation from ruminants using tannins and saponins. Trop Anim Health Prod. 2012;44:729–39.
- Guan H, Wittenberg KM, Ominski KH, Krause DO. Efficacy of ionophores in cattle diets for mitigation of enteric methane. J Anim Sci. 2006;84:1896–906.
- Haque, M. Dietary manipulation: a sustainable way to mitigate methane emissions from ruminants. J Anim Sci Technol 60, 15 (2018). https://doi.org/10.1186/s40781-018-0175-7
- McAllister TA, Newbold CJ. Redirecting rumen fermentation to reduce methanogenesis. Aust J Exp Agric. 2008;48:7–13.
- McSweeney, C. S., B. P. Dalrymple, K. S. Gobius, P. M. Kennedy, D. O. Krause, R. I. Mackie and G. P. Xue. 1999. The application of rumen biotechnology to improve the nutritive value of fibrous feed stuffs: pre and post ingestion. Livest. Prod. Sci. 59:265-283.
- Miles, R. D. and S. M. Bootwalla. 1991. Direct fed microbials in animal production. In: Direct Fed Microbials in Animal Production. Natl. Feed Ingred. Assoc., West Des Moines, IA. 65.
- Mueller-Harvey I (2006) Unravelling the conundrum of tannins in animal nutrition and health. J Sci Food Agric 86(13):2010–2037
- Patra AK, Saxena J. The effect and mode of action of saponins on the microbial populations and fermentation in the rumen and ruminant production. Nutr Res Rev. 2009;22:204–19.
- Pen, B., C. Sar, B. Mwenya, K. Kuwaki, R. Morikawa, and J. Takahashi (2006). Effects of Yucca schidigera and Quillaja saponaria extracts on in vitro ruminal fermentation and methane emission. Anim. Feed Sci. Technol. 129:175– 186.
- Russell JB. Rumen microbiology and its role in ruminant nutrition. Ithaca (NY): Cornell University; 2002.
- Santra, A., and SA Karim. “Rumen Manipulation to Improve Animal Productivity.” Asian-Australasian Journal of Animal Sciences 16, no. 5 (January 1, 2003): 748–63.
- Stewart, C. S., G. Fonty and Ph. Gouet. 1988. The establishment of rumen microbial communities. Anim. Feed Sci. Technol. 21:69-97.
- Tamminga, S., A. Bannink, J. Dijkstra and R. Zom. 2007. Feeding strategies to reduce methane loss in cattle. Report 34, Animal Science Group. http://edepot.wur.nl/28209.
- Trombetta, D., Castelli, F., Sarpietro, M. G., Venuti, V., Cristani, M., Daniele, C., et al. (2005). Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 49, 2474–2478. doi: 10.1128/AAC.49.6.2474-2478.2005
- Wallace RJ. Antimicrobial properties of plant secondary metabolites. Proc Nutr Soc. 2004;63:621–9. 50.
- Zhang, J.; Xu, X.; Cao, Z.; Wang, Y.; Yang, H.; Azarfar, A.; Li, S. Effect of Different Tannin Sources on Nutrient Intake, Digestibility, Performance, Nitrogen Utilization, and Blood Parameters in Dairy Cows. Animals 2019, 9,507. https://doi.org/10.3390/ani9080507








1 Trackback / Pingback