Abstract
Pasteurella multocida is a mysterious pathogen causing considerable economic losses in swine industry. It’s notable for the variety and number of distinct illness syndromes it’s linked to, as well as the wide range of host species it affects. Disease causes by bacterium in grower and finisher pigs are progressive atrophic rhinitis, pneumonic pasteurellosis and sepsis. Main clinical finding associated in porcine pasteurellosis include deviation of snout, retarded growth, dyspnoea and bluish discoloration of skin. P. multocida is the most common zoonotic isolate found in human diseases, and it is transmitted to people through animal bites or contact with nasal secretions. Immunocompromised person who engaged in piggery are particular high risk of acquiring infection from swine. Diagnosis can be done by isolation of organism from clinical specimen in blood agar or brain heart infusion agar. Prevention can be by means of public awareness and establishing surveillance system to early detection P. multocida from swine herd.
Keywords: Pig, Pasteurellosis, Zoonosis, Histopathology, Immunohistochemistry
Introduction
In a world where most emerging and re-emerging infectious diseases are zoonotic in nature and our contacts with both domestic and wild animals abound, there is growing awareness of the potential for human acquisition of animal diseases. Pasteurella multocida is recognized as an important veterinary pathogen and having zoonotic potential, which causes diseases in a wide range of animal species. It is the causative agent of numerous, economically important disease, including avian fowl cholera, haemorrhagic septicemia, enzootic pneumonia and swine atrophic rhinitis. In human it causes localized abscess and also associated with acute or chronic respiratory disease so it regard as occupational pathogen (Wilson and Ho, 2013). P. multocida has been recognized as a pathogen of respiratory disease in swine over 125 years ago contributing significant health hazard to swine industry. This pathogen is widespread in several pig rearing countries of the world including India. There is increasing trend in the outbreak of Pasteurellosis in pigs of India causing a huge economic loss to the farmers. It is a common commensal in the nasopharynx of pigs (Buddle, 1985) and considered as important secondary invaders in pneumonia after the initiation of the diseases by viruses or mycoplasmas. This pathogen is the established cause of progressive atrophic rhinitis (PAR), suppurative bronchopneumonia and rarely-seen haemorrhagic septicaemia that pose a major threat to pig-rearing industry throughout the world (Chanter and Rutter, 1989). Pneumonic Pasteurellosis caused by P. multocida belonging to Pasteurellosis family, is an economically important global pathogen of swine associated with high morbidity and mortality. Out of its all 5 serotypes (A, B, C, D, E), (Serotype A & D) are responsible for causing frequent mortality in pigs. In India, mainly P. multocida belonging to capsular type D followed by A is responsible for causing mortality in pigs due to respiratory distress (Rajkhowa et al., 2012; Thakor et al., 2019). Previously P. multocida was considered opportunistic pathogens but Filho et al. (2015) concluded that P. multocida can also act as primary pathogen in pneumonia and septicemic pasteurellosis in pigs rather being regarded as opportunistic secondary pathogen. Now day’s involvements of the nervous system also reported in naturally infected pigs with P. multocida which indicative newer aspects of pathogenesis (Sahoo et al., 2020).
History
The genus Pasteurella was originally proposed and described by Trevisan in 1887. It consisted of a group of nonmotile, small (0. 7 µm by 0. 5 µm), Gram-negative coccobacilli often exhibiting a characteristic type of bipolar staining. The organism was first isolated by Parroncito in the year 1879 and it was Louise Pasteur who described the species and documented it as the cause of Fowl cholera in 1880. The generic name Pasteurella was suggested by Trewisan in 1887 and in 1900 Lignieres added specific name for each organism according to species specificity. In 1939, Rosenbusch and Merchant proposed the alternative name as P. multocida (Balows and Duerden, 1998).
Pneumonia was apparently recognized as an important disease in swine in antiquity by Aristotle (circa 343 BC) who described three diseases of swine; one disease chiefly involved the jaws and windpipe but extended to and strained the lungs, followed by death. The work of Bollinger, Hueppe, Kitt and Nocard incriminated P. multocida as an important aetiology of swine pneumonia about 125 years ago (Hayes, 1908).
Microbiology
- multocida is a small, pleomorphic, Gram-negative, nonflagellated coccobacillus. Microscopic analysis of fresh cultures or clinical specimens using Leishman’s stain, methylene blue, or Giemsa stain shows bipolar-staining rods. P. multocida isolates are aerobic or facultative anaerobic and grow well at 37°C on 5% sheep’s blood (the preferred culture medium) in dextrose-starch, casein-sucrose-yeast (CSY), chocolate, Mueller-Hinton, or brain heart infusion (BHI) agar (OIE, 2012); however, there is no growth on MacConkey agar. On blood agar plates it forms grayish, nonhemolytic colonies, often mucoid, with a characteristic “sweetish” odor. It does not grow on MacConkey agar, is oxidase and catalase positive, and produces indole. Most clinical isolates are catalase, oxidase, indole, and ornithine decarboxylase positive. Most isolates also ferment sucrose, glucose, and maltose. Media containing vancomycin, clindamycin, gentamicin, neomycin, kanamycin, and/or amikacin, either singly or in combination, have been used to select for Pasteurella (Smith & Baskerville, 1983).
Serotypes of P.multocida in pigs
P.multocida strains are currently classified into five capsular serogroups (A, B, D, E, and F) and 16 Heddleston lipopolysaccharide (LPS) serovars (1–16). Out of its 5 serotypes, serotypes A and D were frequently reported for causing mortality in pigs (Liu et al., 2017). Capsular type A (P. multocida type A) is one of the most common agents associated with bronchopneumonia in pigs and type D is associated with atrophic rhinitis and pneumonia (Paladino et al., 2017).
Epidemiology
- multocida has been isolated throughout the world from a wide array of wild and domesticated mammals and birds, including aquatic mammals (Smith et al., 1978). It causes acute or chronic disease of importance in poultry, cattle, water buffalo, swine, sheep, and rabbits. P. multocida often occurs as part of the normal flora, and clinically inapparent infections that spark disease outbreaks in immunologically naïve animals are common in many hosts. It has no known environmental reservoir. Pasteurellosis in pigs is now recognized worldwide, with cases reported in China, Serbia, New Zealand, Canada, United States, Brazil, Denmark, Taiwan, Argentina and India causing significant economic loss to swine industry (Yeh et al., 2017).
Table 1: Clinical manifestation of Pasteurella multocida in different animals
| Host | Disease | Serotypes |
| Cattle & Buffaloes | Haemorrhagic septicaemia
Bovine pneumonic pasteurellosis |
Pasteurella multocida B:2 and E:2 |
| Sheep & Goat | Pneumonic pasteurellosis | Pasteurella haemolytica A’2 and T |
| Pigs | Atrophic Rhinitis, Pneumonia, Septicaemia | Pasteurella multocida A and D |
| Rabbit | Snuffles | Pasteurella multocida A and D |
| Poultry | Fowl Cholera | Pasteurella multocida A, D and F |
| Human | Localized abscess | – |
Transmission
Transmission is through direct contact with nasal secretions, where a chronic infection ensues in the nasal cavity, paranasal sinuses, middle ears, lacrimal and thoracic ducts of the lymph system, and lungs. Preexisting or coinfection with other respiratory pathogens, particularly Bordetella bronchiseptica or Mannheimia haemolytica significantly enhances colonization by P. multocida, leading to more severe disease. Environmental conditions, stress, and the overall health of the animal also appear to play important roles in disease severity and likelihood of transmission (Register and Brockmeier, 2019). Bacterium may occasionally be spread via aerosols; nose‐to‐nose contact is the common route of infection. Introduction of P. multocida into a herd is usually by introduction of infected swine. Spread within a herd occurs rapidly both vertically from infected dam to suckling piglets and horizontally between infected and uninfected animals (Fablet et al., 2011). Spread related to contaminated fomites or intermediate hosts has been suggested (Desrosiers, 2011), and there is some evidence consistent with occasional interspecies transmission of avian, bovine, ovine, and porcine strains (Davies et al., 2004).
Porcine pasteurellosis
- Atrophic Rhinitis: Atrophic Rhinitis (AR), a syndrome which may include facial distortion, turbinate bone destruction and retarded growth of young pigs, has long been recognised as a common condition of domesticated pigs in all countries. The clinical effects described are attributed to a so-called dermonecrotoxin, often referred to as PMT (for ‘‘Pasteurella multocida toxin’’), which is associated with certain strains of multocida, mostly capsular type D, but other capsular types may also produce PMT (Wilkie et al., 2012). The main clinical signs of AR include distorted maxillae, deviation of nasal septum, erosion or absence of nasal turbinates, and possibly, depressed growth rate, though the latter effect may be associated with management practices more than the disease. Infections acquired after growth is complete are unlikely to cause any facial deformities. The lesions of bony tissue are permanent, although the mucosa may repair. Most pigs are slaughtered at a relatively young age, so it is not known what proportion, if any, eventually eliminate the toxigenic strains from their oropharyngeal tissues.
- Pneumonia: Pneumonic pasteurellosis most commonly occurs in grower‐ and finisher‐age swine where it exacerbates porcine respiratory disease complex (PRDC) that is initiated by one or more primary respiratory pathogens. Clinical signs can vary depending on the pathogens involved and stage or severity of the disease but often include coughing, intermittent fever, depression, anorexia, reduced growth rate, labored breathing or thumping, and in severe cases cyanosis or blue discoloration, especially in the tips of the ears. Macroscopic lesions include typical red‐to‐gray firm consolidation with a cranial ventral well‐demarcated lobular distribution and purulent exudate in lumens of conducting airways and alveoli characteristic of acute to chronic bacterial bronchopneumonia. Microscopic lesions consist of suppurative bronchopneumonia characterized by neutrophil infiltration of bronchial and alveolar spaces and interstitial thickening (Pors et al., 2011).
- Septicemic pasteurellosis: Septicemic pasteurellosis typically has a sudden onset and acute progression of disease with high morbidity and mortality. Clinical signs include high fever, severe dyspnea, cyanosis of the ventrum and ears, anorexia, weakness, and prostration. One of the most striking features is edema and hemorrhage of the ventral neck that may become necrotic (Ujvári et al., 2015). Subcutaneous hemorrhagic edema is often seen in cases involving capsular type B strains (Cardoso‐Toset et al., 2013; Ujvári et al., 2015). Other lesions associated with septicemic pasteurellosis include edema of the pharynx and trachea, pulmonary edema and hemorrhage with or without pulmonary consolidation, enlarged edematous and hemorrhagic lymph nodes, and congestion and hemorrhage of the visceral organs, occasionally with fibrin deposition in the peritoneal and pleural cavities (Mackie et al., 1992). Microscopically, intravascular thrombosis and changes consistent with widespread vascular damage, such as edema, hemorrhage, and necrosis, are present in tissues, and bacteria can often be observed in the vessels and throughout affected tissues.
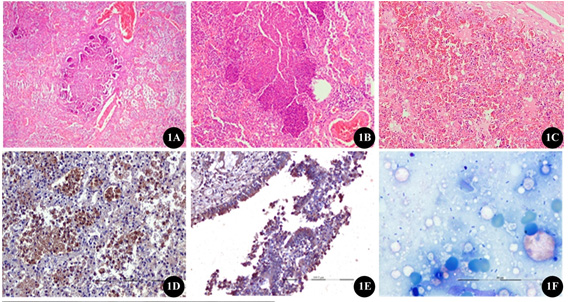
(1A) Section of lungs tissues from pigs naturally infected with P. multocida showing the presence of necropurulent exudates in the bronchiolar lumen with marked infiltration of macrophages within the alveoli (H&E x40);
(1B) higher magnification of the same showing the presence of oat cells admixed with abundant fibrin with necrotic cellular debris interspersed with bacterial colonies within the alveoli and bronchioles(H&E x100);
(1C) Lungs showing severe congestion, oedema and haemorrhages throughout the entire section (H&Ex100);
(1D) intense cytoplasmic immunolocalization of Pasteurella multocida antigen in the alveolar macrophages as indicated by brown signals using mouse polyclonal anti-P. multocida hyperimmune serum (IHC-DAB-x200);
(1E) The bronchiolar epithelium and the inflammatory exudates showing strong cytoplasmic immunoreactivity for P. multocida antigenas indicated by brown signals (IHC-DAB-x100);
(1F) Giemsa stained impression smear of lungs showing bipolar organism suggestive of P. multocida (Thakor et al., 2019).
Public Health significance
multocida is an important zoonotic agent and is responsible for most human infections related to animal bites or scratches. Although P. multocida is an awesome pathogen for young cattle and birds, it may occur as a relatively benign member of the nasopharyngeal microflora of adult cattle, rabbits, cats, and dogs which do not usually develop severe pulmonary infections. Humans do not usually develop acute pulmonary disease, although there have been reports of a mild pasteurellosis in some cattle handlers. It seems likely that this organism can colonize normal human nasopharyngeal membranes.
Most human infections with P. multocida occur as localized abscesses of the extremities or face as a result of cat or dog bites but infection following bites from pigs, rabbits, rats, and various wild animals has been reported (Wilson and Ho, 2013). Historically, such infections were first associated with tiger bites in India. Domestic cats, as well as exotic felines, carry P. multocida as part of their normal gingival microflora; organisms introduced into human tissues as a result of bites and scratches enter the subcutaneous tissues, quickly producing severe local abscesses, which eventually spread to the draining lymph nodes. These abscesses require surgical drainage due to their extensive edema and fibrosis (which also reduces the effectiveness of chemotherapeutic intervention, although this organism is susceptible to most antibiotics in vitro).
multocida is not a usual constituent of the human upper respiratory tract, but strains genetically identical to those found in swine are frequently isolated from pig farmers and from inhabitants of regions with intensive pig breeding (Marois et al., 2009). Water from scalding tanks is a potential source of exposure for abattoir workers (Marois et al., 2008). Most human carriers remain healthy, but P. multocida may also be associated with acute or chronic respiratory disease. It has been proposed that pneumonic pasteurellosis be considered an occupational disease. Appropriate precautions should be observed by immunocompromised persons who have contact with swine infected with P. multocida.
Diagnosis
A definitive diagnosis of PAR depends on clinical and pathological observations and demonstration of toxigenic P. multocida. Lung lesions caused by P. multocida are not pathognomonic and cannot be used as the only criteria to establish a definite diagnosis. The history of the outbreak, histopathology, and isolation of the organism should be used to confirm the original presumptive diagnosis. P. multocida grows readily on blood agar, so it can be used for the isolation of organism. A multiplex capsular PCR (Townsend et al., 2001) also used for detection of nucleic acid in clinical specimen.
Prevention and control
- During an outbreak, one should resort to immediate whole herd vaccination, irrespective of previous vaccination history.
- The use of either broth bacterin or oil adjuvant vaccine is recommended. Sanitary measures include early detection and isolation of new cases and their immediate treatment with antibiotics, deep burial of carcasses or incineration, and the prevention of movements of animals to disease-free areas.
- In endemic areas the prevention measures include, vaccination on a routine prophylactic basis, preferably two to three months before the high-risk season (monsoon), awareness of the disease among farmers backed up by a good disease reporting/disease information system, segregation of animals from endemic and non-endemic areas to avoid contact with carriers (Benkirane and Alwis, 2002).
- To reduce vertical transmission from infected dams to suckling offspring, the sow’s feed can be medicated during the final month of gestation. Sulfonamides and tetracyclines are most widely used.
- All‐in/all‐out systems are favoured for farrowing, weaner, and preferably fattener units.
- Stocking density should be reduced, strict hygiene measures should be implemented, and ventilation rates should be maintained to reduce airborne bacteria, noxious gases, and dust.
Conclusion
Porcine pasteurellosis is devastating disease confronting high morbidity and mortality in pigs. It is highly prevalent in Indian pigs so care should be taken while handling of pigs to prevent the zoonotic spillover because P. multocida having zoonotic potential. Disease in human occurs through bite of animals and also having cross species transmission. The newer aspect of pathology that is involvement of nervous system in pig requires examining brain while conducting post-mortem examination of suspected carcass of pigs. Infection in pig farm can be control by continuous monitoring and surveillance programme. Disease animal should be treated with suitable broad spectrum antibiotics. Clinician and microbiologist should be aware of zoonotic importance of P. multocida.
References
- Balows, A. and Duerden, B.I. 1998. Systematic bacteriology, vol. 2. Topley & Wilson’s microbiology and microbial infections. Oxford University Press, New York: 106.
- Benkirane, A. and De Alwis, M.C.L., 2002. Haemorrhagic septicaemia, its significance, prevention and control in Asia. VETERINARNI MEDICINA-PRAHA-, 47(8), pp.234-240.
- Buddle JR. 1985. Bacterial and Fungal Diseases of Pigs, Australian Bumu of Animal Health/Australian Government Publishing Service, Canberra. Animul Heulth in Australia. 6: 133.
- Cardoso-Toset, F., Gómez-Laguna, J., Callejo, M., Vela, A.I., Carrasco, L., Fernández-Garayzábal, J.F., Maldonado, A. and Luque, I., 2013. Septicaemic pasteurellosis in free-range pigs associated with an unusual biovar 13 of Pasteurella multocida. Veterinary microbiology, 167(3-4), pp.690-694.
- Chanter, N. and Rutter, J.M. 1989. Pasteurellosis in pigs and the determinants of virulence of toxigenic Pasteurella multocida. Pasturella and pasteurellosis/edited by C. Adlam, JM Rutter.
- Davies, R.L., MacCorquodale, R. and Reilly, S., 2004. Characterisation of bovine strains of Pasteurella multocida and comparison with isolates of avian, ovine and porcine origin. Veterinary microbiology, 99(2), pp.145-158.
- Desrosiers, R., 2011. Transmission of swine pathogens: different means, different needs. Animal health research reviews, 12(1), pp.1-13.
- Fablet, C., Marois, C., Kuntz-Simon, G., Rose, N., Dorenlor, V., Eono, F., Eveno, E., Jolly, J.P., Le Devendec, L., Tocqueville, V. and Quéguiner, S., 2011. Longitudinal study of respiratory infection patterns of breeding sows in five farrow-to-finish herds. Veterinary microbiology, 147(3-4), pp.329-339.
- Hayes MH. 1908. Translation of Friedberger and Frohner’s Veterinary Pathology, Vol. 2. London: Hurst and Blackett Ltd, pp. 274–285.
- Liu, H., Zhao, Z., Xi, X., Xue, Q., Long, T. and Xue, Y., 2017. Occurrence of Pasteurella multocida among pigs with respiratory disease in China between 2011 and 2015. Irish veterinary journal, 70(1), pp.1-6.
- Mackie, J.T., Barton, M. and Kettlewell, J., 1992. Pasteurella multocida septicaemia in pigs. Australian veterinary journal, 69(9), pp.227-228.
- Marois, C., Cariolet, R., Morvan, H. and Kobisch, M., 2008. Transmission of pathogenic respiratory bacteria to specific pathogen free pigs at slaughter. Veterinary microbiology, 129(3-4), pp.325-332.
- Marois, C., Fablet, C., Gaillot, O., Morvan, H., Madec, F. and Kobisch, M., 2009. Molecular diversity of porcine and human isolates of Pasteurella multocida. Journal of applied microbiology, 107(6), pp.1830-1836.
- OIE, World Organisation for Animal Health. 2012, posting date. Manual of diagnostic tests and vaccines for terrestrial animals. http://www.oie.int/international-standard-setting/terrestrial-manual/access-online.
- Oliveira, J.X.D., Morés, M.A., Rebelatto, R., Agnol, A., Plieski, C.L., Klein, C.S., Barcellos, D.E. and Morés, N., 2015. Pasteurella multocida type A as the primary agent of pneumonia and septicaemia in pigs. Pesquisa Veterinária Brasileira, 35, pp.716-724.
- Paladino, E.S., Gabardo, M.D.P., Lunardi, P.N., Morés, N. and Guedes, R., 2017. Anatomopathological pneumonic aspects associated with highly pathogenic Pasteurella multocida in finishing pigs. Pesquisa Veterinária Brasileira, 37, pp.1091-1100.
- Pors, S.E., Hansen, M.S., Bisgaard, M. and Jensen, H.E., 2011. Occurrence and associated lesions of Pasteurella multocida in porcine bronchopneumonia. Veterinary microbiology, 150(1-2), pp.160-166.
- Rajkhowa, S., Shakuntala, I., Pegu, S.R., Das, R.K. and Das, A., 2012. Detection of Pasteurella multocida isolates from local pigs of India by polymerase chain reaction and their antibiogram. Tropical animal health and production, 44(7), pp.1497-1503.
- Register, K.B. and Brockmeier, S.L., 2019. Pasteurellosis. Diseases of swine, pp.884-897.
- Sahoo, M., Baloni, S., Thakor, J.C., Dinesh, M., Bhutediya, J., Qureshi, S., Dhama, K., Dubal, Z.B., Singh, K. and Singh, R., 2020. Localization of Pasteurella multocida antigens in the brains of pigs naturally infected with Pasteurellosis revealing a newer aspect of pathogenesis. Microbial pathogenesis, 140, p.103968.
- Smith IM, Baskerville AJ. 1983. A selective medium for the isolation of multocida in nasal specimens from pigs. Br Vet. J. 139:476–486.
- Smith, A.W., Vedros, N.A., Akers, T.G. and Gilmartin, W.G., 1978. Hazards of disease transfer from marine mammals to land mammals: review and recent findings. Journal of the American Veterinary Medical Association, 173(9), pp.1131-1133.
- Smith, I.M. and Baskerville, A.J., 1983. A selective medium for the isolation of multocida in nasal specimens from pigs. British Veterinary Journal, 139(6), pp.476-486.
- Thakor, J., Dinesh, M., Baloni, S., Bhutediya, J., Qureshi, S., Singh, K.P. and Sahoo, M., 2019. Role of apoptosis in the pathology of pneumonic pasteurellosis in naturally infected pigs. Indian Journal of Veterinary Pathology, 43(1), pp.56-60.
- Ujvári, B., Szeredi, L., Pertl, L., Tóth, G., Erdélyi, K., Jánosi, S., Molnár, T. and Magyar, T., 2015. First detection of Pasteurella multocida type B: 2 in Hungary associated with systemic pasteurellosis in backyard pigs. Acta Veterinaria Hungarica, 63(2), pp.141-156.
- Wilkie, I.W., Harper, M., Boyce, J.D. and Adler, B., 2012. Pasteurella multocida: diseases and pathogenesis. Pasteurella multocida, pp.1-22.
- Wilson, B.A. and Ho, M., 2013. Pasteurella multocida: from zoonosis to cellular microbiology. Clinical microbiology reviews, 26(3), pp.631-655.
- Yeh JC, Lo DY, Chang SK, Chou CC, Kuo HC.2017. Antimicrobial susceptibility, serotypes and genotypes of Pasteurella multocida isolates associated with swine pneumonia in Taiwan.Vet Rec: e 1-7.
| The content of the articles are accurate and true to the best of the author’s knowledge. It is not meant to substitute for diagnosis, prognosis, treatment, prescription, or formal and individualized advice from a veterinary medical professional. Animals exhibiting signs and symptoms of distress should be seen by a veterinarian immediately. |










Be the first to comment