Productivity is the key to growth and reproduction is backbone of animal production. Reproductive inefficiency is one of the most important causes of economic losses in animal industries and it is realized throughout the world. Despite of remarkable advancement that has been made in the field of reproductive physiology in recent years, infertility due to low conception rate and high embryonic mortality rate remains a major problem. To meet future needs and to be able to sustain agricultural production, agricultural research and its applications there is a need to use all emerging technologies especially the modern reproductive biotechnologies. Development of reproductive techniques like, oestrus synchronization, super ovulation, non-surgical embryo collection, transfer, cryopreservation of embryos, oocytes pick- up from live animals, in vitro maturation, fertilization, embryo development and cloning could not make an impact on quality animal production due to non availability of low cost embryos from quality animals. There is a scope for further improvement in reproductive status of our farm animals. The recent scientific developments in assisted reproductive techniques have made it possible to manipulate the reproductive processes in many ways to revolutionize world animal agriculture.
In Vitro Fertilization
In Vitro Fertilization (IVF) is a method of assisted reproduction to increase the possibility of pregnancy. As the term ‘in vitro fertilization’ explains itself, it is the procedure to fertilize eggs with sperm outside the body.
History
- In vitro fertilization (IVF) is a fertility procedure which first succeeded as recently as 1978 by Dr. Edwards (an embryologist) and Dr. Steptoe (a gynaecologist) in England. Since then the technology has been further refined and developed by physicians and embryologists, with over 20,000 babies born worldwide.
- The first in-vitro fertilization to produce test tube baby “Durga” in India and second in the world was performed by a Calcutta based doctor Dr. Subhash Mukhopadhyay on October 3, 1978.
- World’s first buffalo calf ‘Pratham’ was born through in vitro maturation and in vitro fertilization of buffalo oocytes developed at NDRI.
- The first attempt to use in vitro matured bovine oocytes occurred over 25 years ago when fertilization was achieved using a rabbit oviduct to capacitate bull spermatozoa. However suitable culture conditions were later established that allow for IVM, IVF and IVC of bovine embryo resulting in acceptable embryonic growth rates.
- The first ruminant offspring from in vitro fertilization was a bull calf born in 1981 .Since then fertilization has been accomplished in vitro in many species. Brackett et al.(1982) use in vivo matured oocytes for IVF system and produce first calf in the world.
- Lu et al. (1987) from Ireland developed method of transvaginal ultrasound guided aspiration technique of OPU.
- In Canada, using a laparoscopic technique to recover secondary oocytes, Lambert et al. (1983) succeeded in producing six IVF calves.
- The first calves born after IVF of artificially matured oocytes were those reported by Handa et al. (1986) from Japan.
- The in vitro maturation (IVM) ,in vitro fertilization (IVF) and in vitro culture (IVC) of cattle oocyte are valuable tools that can be easily applied for both research and agriculture purpose as described by Gordon (1994) .
- The number of embryos that develop to the blastocyst stage invitro per 100 cumulus oocyte complexes (COCs) is substandard to that for in vivo production system (Sirard and Lambert,1986).
- Sheep were the first of the farm animals to be used in IVF studies by Dauzier & Thibault in France during 1950.
- The first foal from IVF was born in France on June 14, 1990.
Steps for IVF in cattle
- Oocyte (Egg) Collection
- In vitro maturation
- Sperm capacitation
- In vitro Fertilization
- Embryo Transfer
(1) Collection of oocytes
- Live animal – Ovum pick-up method
- Slaughtered animal – a) Slicing of ovaries b) Aspiration
Slaughtered animal
a) Oocyte aspiration
Ovaries collected from slaughtered animals then the aspiration method is convenient method of application. Aspiration of oocyte is done using an 18- gauge needle attached to a 10 ml syringe to avoid disruption of the surrounding cumulus cells. For livestock the use of plastic disposable syringe is acceptable.
b) Slicing of ovaries –
The ovaries are sliced in order to retrieve oocytes. It enhances the retrieval of a larger number of oocytes compare to the dissection and aspiration. A higher number of oocytes recovered per ovary were by slicing and dissection then that of aspiration. However, it require a longer period of retrieval and is not advisable when manipulating large number of ovaries because the extended period of oocyte retrieval would decrease the quality of oocytes and lower the in vitro maturation potential of oocytes.
From Live Animal
a) By Endoscopy –
The earliest report on oocyte recovery from the live cow came from North America, where the recovery of in vivo matured oocyte by way of an endoscope inserted through the skin of the right Para lumber fossa.
b) By the Laparoscopy – The surgical operation of flank region & assess to the ovary, ultimately to ovum.
Trans vaginal USG guided follicle aspiration
The technique of transvaginal USG guided follicle aspiration was first described in human by Wikland and their co-workers in 1987. This use in non-stimulating cows. Ovum pick-up was performed using an ultrasound machine with a transvaginal convex transducer (5MHz) with single lumen 19 gauge 60 cm long needle guide and vacuum pressure of 90 mm Hg. It can be performed at any stage of the estrous cycle, two times weekly repeated.
Oocyte selection
Oocytes with compact multilayered cumulus cells and evenly granulated cytoplasm are selected for in vitro maturation and physical contact between oocyte and cumulus cells has been considered necessary for the transfer of nutrient and essential for oocyte development. Oocytes classification was carried out in Petri dishes with krebs medium and the oocytes obtained were classified into three classes
Category-1 (good): Oocytes with homogenous granulate ooplasm. The cumulus cells cover the oocyte with a compact multi layer coat.
Category-2 (fair): Oocytes with a homogenous granulate ooplasm. The cumulus cells cover the oocyte with a coat of three to four layers.
Category-3 (poor) – The cumulus cells are visibly disrupted and create a cumulus cloud or naked oocyte.
(2) In vitro Maturation
Collected oocytes are placed in culture-medium-filled dishes and allowed a period of about 24 hours to reach maturity. These dishes are incubated at about 38.5 degrees Celsius. (Normal cow body temperature) In vitro Maturation media – a) Complex medium, b) Simple medium
Media used for in vitro Maturation
Simple media-These are usually bicarbonate buffered systems containing basic physiological saline with pyruvate, lactate and glucose. Media supplemented with serum or albumin and trace amount antibiotic added.
Complex media – It contain amino acid, vitamins, purines and other substances, mainly at levels at which they are found in serum and fixed nitrogen is present in free amino acid. Most widely use Tissue culture medium 199 (TCM-199) with Earle’s salt.
(3) Sperm Capacitation
For capacitation frozen semen is used and percoll based separation system is the most common method for isolating the motile sperm fraction after thawing. Although other systems can be used like swim-up, simple centrifugation but separation through a percoll gradient offers the consistency, flexibility and reliability. Bovine sperm capacitated efficiently in vitro by exposure for 4 hour in heparin.
(4) Invitro Fertilization
Successful in vitro fertilization requires prior maturation of participating oocytes as well as appropriate preparation of sperm. Mammalian spermatozoa are not immediate capable of fertilizing oocytes because they must undergo a period of preparation which normally occurs in the female reproduction tract. The number of spermatozoa is very important, if it is less in number result in reduce rate of fertilization and higher sperm number result in the polyspermy. After twenty-four hours in IVM medium:
- The oocytes are washed and moved into new dishes with IVF culture medium.
- Sperm are added at a concentration optimal for sperm capacitation and fertilization.
- These dishes are then incubated for 18 hours to allow fertilization to occur.
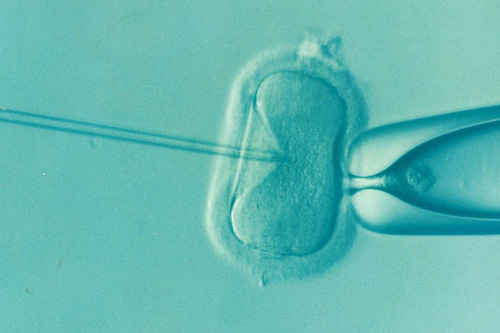
Intracytoplasmic Sperm Injection
ICSI is a highly technical procedure designed to insert a sperm into the cytoplasm of egg using a thin glass tube under the special microscope with a manipulator. When a bull sperm encounters an oocyte in the oviduct of the live cow, an intricate surface reaction is triggered which result in activation of the oocyte. Activation leads to completion of the second meiotic division formation of female pronucleus, release of cortical granule contents.
IntracytoplasmicInjection
Invitro Culture – After eighteen hours in IVF medium:
- The dishes are checked for sperm hyper activation.
- The zygotes (1-cell embryos) are washed and moved to IVC medium.
- Then dishes are incubated for about 7 days and are checked periodically for embryo cleavage (cell division).
(5) Embryo Transfer
On about day seven after fertilization, the embryos (at the blastocyst stage) are transferred non-surgically to recipients. The recipients are synchronized to ensure timing of embryo placement post ovulation. Care is taken to place embryos in the uterine horn on the same side as the corpus luteum (where ovulation occurred) to facilitate maternal recognition of pregnancy.
Evaluation of embryo development
- 2-16 cell stages
- Morula : Embryo with >32 cell stage
- Compact morula : Compaction of blastomeres
- Early blastocyst: Formation of blastocoel had just started
- Blastocyst: Well defined blastocoels & start differentiation
- Expanded blastocyst : Expanded with zona thinning
- Hatched blastocyst :Partly or completely come out from ruptured zona
Uses of IVF
- Twin calves from beef cows
- Single calves from dairy cows
- IVF as an adjunct of conventional cattle ET
- Genetic salvage service
Limitation in Buffalo
Limitation in buffalo IVM, IVF and IVC in terms of lower yield of useable oocytes , high rate of follicular Atresia , low fertilization rate, seasonal effect on follicular recruitment and quality oocytes, seasonal effect on quality of semen and the quality of cattle that are slaughtered. This will have to be taken in to consideration while performing the IVF in buffalo.
Sperm sexing
Manipulating the sex of offspring has been a dream of the cattle production industry for decades. The desire to manipulate sex is for obvious (females are required for milk production and males grow faster) and not so obvious (the purebred industries of both dairy and beef desire more females than males) reasons. It would be most efficient to control sex at the time of conception so all offspring would be of the desired sex.
Idea of sexed semen started with the early Greeks when Democritus, 470-402 BC, suggested that the right testis produced males. Whereas the left testis produced females. Many theoretical differences between the male and female producing a sperm have been suggested, including physical differences such as size, weight and density, swimming speed, electrical surface charges, surface macromolecular proteins, differential effects of pH and differing effects of atmospheric pressure. Some methods were tried practically viz. Gradient swim-down procedure using albumin based on Y chromosome bearing spermatozoa is smaller thus exhibit faster downwad swimming than X spermatozoa, surface antigenic difference i.e. H-Y antigen, sex specific proteins on sperm surface, free sorting based on volumetric difference of sperm heads with X being larger than Y, centrifugal countercurrent distribution using an aqueous two-phase system, genetic approaches, fluorescence in situ hybridization, quantitative PCR, sort raanalysis and the oldest one was Quinacrine mustard method. Details of much of these had discussed by Amann and Seidel. But all these failed due to or the other reasons like they suffer from low accuracy, too time consuming, damage tbo sperm making them infertile, poor repeatability, lack of suitable scale-up procedures, or other problems preventing their commercialization. The one and only flow cytometry is found to be effective with consistent result of high efficacy (85-95 %) in different mammalian species.
Applications of sexing of semen:
- The main application of sexed sperm to date has been to breed dairy heifers to produce female calves
- Conservation of zoo and other wild animals like threatened or endangered animal
- In livestock species, the sex of an animal is arguably the most important genetic trait with considerable influence on profit.
- Captive breeding programme there is usually a preference for the production of female offspring because these will be most useful for long-term management of a breeding population and preventing inbreeding
- To control the sex of offspring of endangered and exotic animals is obvious: to minimize inbreeding within captive populations
- Mimicking those sex ratios occurring in the wild: e.g. for species having female dominated social group (e.g. gorillas)
- For populations in which one sex has more intrinsic value
Flow cytometry method
Till date the method is costly, has larger wastages of semen and reduced fertility of sorted semen but still the only reliable method with the efficacy of 80-95 %. The method is based on the differences in the amount of DNA present in X and Y chromosome bearing spermatozoa. The former being larger contain 3-4 % more DNA than later. This flow cytometric sex-sorting of asperm according to their DNA content is known as the Beltsville Sperm Sexing Technology and was patented by the USDA, with Dr. Lawrence Johnson as the inventor.
Flow cytometry is a method of measuring multiple physical and chemical characteristics of particles by optical means. Flow cytometry is a laser-based technology that permits a rapid analysis of specific physical and chemical properties of cells or other biological or non-biological particles. It is a powerful technique as it provides real time quantitative information on a cell by cell or particle by particle basis. Sophisticated data analysis makes it possible to quantify information from single cells up to millions of cells after a single sample run out of one test tube
Detailed procedures for semen sorting are not possible to provide in text. However, an overview will be provided.
Stained sperm with fluid are pumped with pressure towards nozzle. In nozzle this fluidic stream containing semen is brocken into droplets of 70 mocron (@ 70,000-80,000 droplets/sec) by vibration produced by piezo-electric crystals. Thus the droplets formed are of different types: droplets containing no spermatozoa, droplet with single spermatozoa, droplet containing no sperm, more than one spermatozoa, damaged sperm, membrane compromised sperm, or sperm that are indistinguishable relative to DNA content droplet with other cells, debris, parts of sperm head, tail etc. Further droplets with X or Y may be with live or dead spermatozoa. On, laser exposure only the droplet with live spermatozoa (H33342 stained) fluoresce blue and not the dead spermatozoa (stained by PI/ food dye). This is dead cell gating effect of flow cytometry. The fluorescence is in proportion to amount of dye (DNA binding) content in spermatozoa with X fluoresce about 4 % brighter. The fluorescence signals are measured by detector (photomultiplier tube) and analyzed by computer. Computer on basis differential fluorescence assign charge (+/-) to each droplet containing spermatozoa; positive charge to Y and negative to X. These were then separated by applying the high voltage electric field. The stream of X- and Y- chromosomes- bearing spermatozoa are collected into tubes containing a 22 % egg yolk Tris extender and the third central stream carrying no charge is rejected as waste. Collection tubes are soaked in BSA solution to overcome the sticking of sperm top the wall of the tube. Test-yolk extender in the collection tube is to provide a concentrated environment for the sorted sperm to swim into. This assists in maintaining viability during the sorting process. Centrifugation of the collected sorted sample is done and the sperm pellet is diluted according to its use.
Limitations:
- Accuracy of 90 % means 10 % chances of error.
- One spermatozoa is being sorted at a time making the system inherently slow and thereby increasing the cost of sorted semen.
- Sexing works better with fresh semen so sorters usually are located near the bull.
- An important point is that about 20 % of the sperm sorted are used for quality control purpose or lost post sorting in the supernatant when they are concentrated by centrifugation.
- Nearly, half –a day is required from ejaculate to process single insemination dose of sexed semen meantime the spermatozoa may undergo some damage like premature acrosomal reaction.
- Lower fertility rate by 20-40 % from conventional AI. However, this difference could be reduced to 10 % if use of sorted limited primarily to well-managed virgin-heifers because of their inherent higher fertility.
- Marginal non-significant higher (1-2 %) rate of pregnancy losses.
- The sperm sexing failed if mother could have role in predetermined the sex of offspring.
- Embryo produced from sorted sperm developed slower and have altered mRNA expression patterns. However, such embryos showed no DNA damaged as evident by Sperm Chromatin Assay of sex-sorted sperm.






Be the first to comment