Introduction
Lactoferrin (LF) is an iron-binding glycoprotein synthesized by specific granules in polymorphonuclear leukocytes and glandular epithelial cells since bacteria require iron for growth, LF can inhibit bacteria by chelating iron under certain conditions. In addition to its iron-binding function, it has been reported that LF may directly kill certain bacterial strains or may weaken bacterial resistance by adhesion to the surface of bacteria. LF in milk seems to play a key role in the defense mechanisms in the mammary gland of dairy cows.
Lactoferrin is one of the protein present in bovine milk. It is also known as “The Red Protein”, it was first identified in milk whey in 1939. Bovine lactoferrin is a metal binding glycoprotein consisting of two globular lobes, each of which contains one iron binding site. Lactoferrin is a component of innate defense system of all animals including humans and usually found in all external secretions, including milk, tears and saliva. This glycoprotein is secreted by mammary epithelial cells. Its concentration varies with stage of lactation of cow, high in colostrum and udder secretions during dry periods. The concentration of lactoferrin is usually increased during intramammary infections and depends upon severity of infections. It is released by secondary granules of neutrophils and epithelial cells of mammary gland in response to inflammatory stimuli. Concentration of lactoferrin in bovine milk and colostrum have relatively low. It varies from 0.02 to 0.35 mg/ml in the milk of healthy lactating cows.
Molecular Structure
Lactoferrin is one of the transferrin proteins that transfer iron to the cells and control the level of free iron in the blood and external secretions. It is present in the milk of humans and other mammals, in the blood plasma and neutrophils and is one of the major proteins of virtually all exocrine secretions of mammals, such as saliva, bile, tears and pancreas.
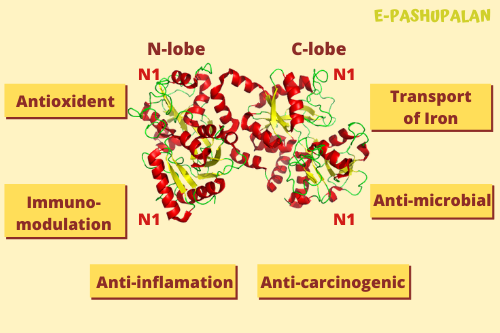
X-ray diffraction reveals that lactoferrin is based on one polypeptide chain that contains about 700 amino acids and forms two homologous globular domains named N-and C-lobes. N-lobe corresponds to amino acid residues 1–333 and C-lobe to 345–692, and the ends of those domains are connected by a short α-helix. Each lobe consists of two subdomains, N1, N2 and C1, C2, and contains one iron binding site and one glycosylation site. The degree of glycosylation of the protein may be different and therefore the molecular weight of lactoferrin varies between 76 and 80 kDa. The stability of lactoferrin has been associated with the high glycosylation degree.
Lactoferrin belongs to the basic proteins, its isoelectric point is 8.7. It exists in two forms: iron-rich hololactoferrin and iron-free apolactoferrin. Their tertiary structures are different; apolactoferrin is characterized by “open” conformation of the N-lobe and the “closed” conformation of the C-lobe, and both lobes are closed in the hololactoferrin.
Each lactoferrin molecule can reversibly bind two ions of iron, zinc, copper or other metals. The binding sites are localized in each of the two protein globules. There, each ion is bonded with six ligands: four from the polypeptide chain (two tyrosine residues, one histidine residue and one aspartic acid residue) and two from carbonate or bicarbonate ions.
Lactoferrin forms reddish complex with iron; its affinity for iron is 300 times higher than that of transferrin. The affinity increases in weakly acidic medium. This facilitates the transfer of iron from transferrin to lactoferrin during inflammations, when the pH of tissues decreases due to accumulation of lactic and other acids. It is demonstrated that lactoferrin is involved not only in the transport of iron, zinc and copper, but also in the regulation of their intake. Presence of loose ions of zinc and copper does not affect the iron binding ability of lactoferrin, and might even increase it.
Functions of Lactoferrin
It shows bacteriostatic activity by binding with iron and bactericidal activity by interacting with bacterium. It can kill susceptible bacteria by a mechanism which is mediated through peptides (lactoferricin).
Mechanism besides its bactericidal activity is presence of lactoferrin receptor on surface of bacteria such as Staphylococcus aureus. Lactoferrin binds with its receptor and promote the activation of bovine complement through an alternative pathway, and thus reduces number of staphylococci in the udder. Lactoferrin regulates inflammatory response by binding to bacterial endotoxin, which inhibits pro-inflammatory cytokines thus indirectly stimulates the immune system. It also inhibits intracellular invasion of pathogens and has immunoregulatory functions that affect antibody synthesis, production of various cytokines, interleukins (IL-1, IL-2 and TNF-a) and complement activation.
In bovines, lactoferrins produced by mammary gland plays dual role, protecting both the mammary gland and neonatal intestine from infection. It has been seen growth and development of gastro intestinal tract in newborn animals fed their dam milk are more rapid than in those fed commercial formula. Interaction of lactoferrin with other proteins such as immunoglobulin G or casein, in nonlactating mammary secretions may affect antimicrobial properties. The fully involuted udder is very resistant to Coliform infections, mostly due to the high content of lactoferrin in secretion. It has been noted exogenous lactoferrin infusion could potentially be usefull in treatment of bovine mastitis and also prevention of mastitis at drying off.
Key action of lactoferrin
- Regulation of iron metabolism, Antibacterial, antiviral, antifungal properties
- Promotion of healthy gut flora, Enhancement of immune function
- Antioxidant and anti-inflammatory effects
Recommended Doses
To servings daily of bovine colostrums concentrations containing the following per servings:
- Immunoglobulin G: 750 mg
- Immunoglobulin A: 45 mg
- Lactoferrin: 100 mg
- Lactoperoxidase: 500 mcg
Conclusion
Lactoferrin with its broad spectrum antimicrobial effect would be a good source of non-antibiotic treatment of udder infection. It has been observed that administration of exogenous lactoferrin during early involution could help in controlling of bacterial growth. It is also known for its priming effect on the innate immunity of the host. In addition, a high concentration of lactoferrin promotes phagocytic activity in the mammary gland and activates bovine complement system. It also regulates the deposition of apoptotic cells and bacteria during the early non lactating period. Keeping in view all these facts about lactoferrin, it can prove to be an ideal non-antibiotic therapy for various udder infections of dairy animals.
References
- Appelmelk, B.J., Yun-qing, A., Geerts, M., Thijs, B.G., Boer, H.A., Maclaren, D.M., Graa¡, J. and Nuijens, J.H., 1994. Lactoferrin is a lipid A-binding protein. Infection and Immunity, 62, 2628-2632
- Reiter, B.,1978. Review of the progress of dairy science: antimicrobial systems in milk. Journal of Dairy Research, 45, 131-147
- Weinberg, E.D., 1978. Iron and infection. Microbiological Reviews, 42, 45-66





Be the first to comment