Introduction
Pigs provide an important source of high-quality protein and production is predicted to increase in future to meet growing global demands for meat. However, the supply of pork is threatened by infectious diseases, and amongst these African swine fever (ASF) is currently causing greatest concern. African swine fever (ASF) is a devastating haemorrhagic fever of pigs that causes up to 100 % mortality (Penrith, Thomson & Bastos, 2004). ASF was first reported in South Africa in 1928. The continuing spread of ASF outside Africa in Europe, the Russian Federation, China and most recently to Mongolia and Vietnam, India has heightened awareness of the threat posed by this devastating disease to the global pig industry and food security. It is listed as a “notifiable disease” by the World Organization for Animal Health (OIE), in part because of its high mortality rate. However, the control of ASF is rely on strict sanitary measures as lack of licensed vaccine and no specific treatment available currently. OIE reported on May 21, 2020, a total of 11 outbreaks in Assam and Arunachal Pradesh states of India, wherein 3701 pigs were died due to ASF. According to the FAO, “its extremely high potential for transboundary spread has placed all the countries in the region in danger and has raised the spectre of ASF once more escaping from Africa. It is a disease of growing strategic importance for global food security and household income”.
African swine fever virus (ASFV)
The causative agent of ASF is ASFV belonging to the genus Asfivirus of the family Asfarviridae. ASFV is the only virus listed in that genus. ASFV is a large double-stranded DNA virus that replicates in the cell cytoplasm and is the only member of the Asfarviridae family. The main target cells for ASFV replication are macrophages. Modulation of macrophage function by the virus is important for the pathogenic and immune evasion mechanisms. The virus genome varies between about 170 and 193 kbp and codes for between 150 and 167 proteins, including those required for virus replication. (Dixon et al., 2013). The virus is known to be highly resistant to low temperatures. ASFV gets heat inactivated at 56°C for 70 minutes or at 60°C for 20 minutes. In addition, it is inactivated by pH <3.9 or >11.5 in serum-free medium.
Host range of ASFV
ASFV infects domestic and wild suids, including warthogs (Phacochoerus africanus) and bushpigs (Potamochoerus larvatus) in Africa and wild boar (Sus scrofa) in Eurasia. Soft ticks of the Ornithodoros species can be infected over long time periods and act as virus reservoirs. ASF is non-zoonotic disease. The role of ticks (Ornithodoros spp.) in transmission of the virus in pigs in India is yet to be established.
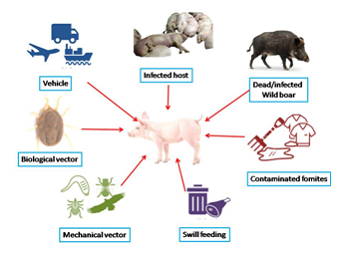
Transmission
Direct transmission
- The entrance of ASFV into pigs normally occurs by direct contact (oronasal) with infected animals,
Indirect transmission
- Ingestion of contaminated animal byproducts, uncooked infected meat (ASFV can remain infectious for 3–6 months in uncooked pork products)
- Contaminated fomites including equipment, vehicles, footwear, feed, or clothing.
Biological vector
- Other routes of transmission are such as cutaneous, subcutaneous, via tick-bites of soft ticks of genus ornithodoros spps.
Traditionally, virus entrance into free regions usually occurs as a result of uncooked pork waste–especially from ships and aircraft–being fed to pigs. Once the disease is established in an area, it mainly spreads by direct contact between sick and healthy animals (domestic pigs and wild Suidae), recovered carrier pigs and soft ticks or, for example, through indirect transmission by lorries, at drinking and eating troughs, via surgical and personal equipment, rodents, or other farm animals.
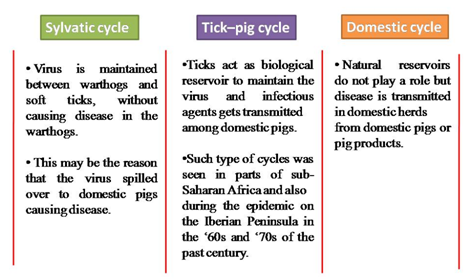
There are three cycles of ASF epidemiology such as sylvatic, tick–pig, and domestic, with addition of more factors, that is, soft Ornithodoros spp. ticks, wild African pigs (mainly warthogs), domestic pigs, and pig-derived products such as pork.
Pathogenesis and Clinical manifestation in susceptible host
Unlike Classical Swine Fever (CSF), which mainly affects young pigs, all age groups are equally susceptible to ASF. ASFV strains are classified as of high, moderate or low virulence. Disease manifestations include peracute, acute, subacute and chronic forms. ASF has an incubation period of 4–19 days. Clinical course lasts for 4–5 days in acute infections or longer in cases of the subacute forms of the disease (Gallardo et al., 2015).
Peracute form
- Pigs die within 4 days post-infection (DPI) after the onset of clinical signs with no evident lesions in organs
Acute form
- High mortality of infected pigs (90–100% mortality) 4–21 DPI
- High fever (40-42°C) with erythema and cyanosis of the skin.
- Vomiting and haemorrhagic diarrhoea may occur.
- Anorexia, cyanosis and incoordination may occur 1–2 days before death.
- Abortion in pregnant sows has frequently been described. Characteristic pathological changes due to vasculitis, including skin erythema, pulmonary oedema, hyperaemic splenomegaly, haemorrhagic lymphadenitis, and petechial haemorrhages in the lungs, urinary bladder and kidneys with an excess of natural fluids in body cavities and spaces.
Subacute form
- moderately virulent isolates are associated with this form of disease
- Clinical signs tend to be less marked
- The mortality may reach upto is 30–70%, with longer incubation period (pigs die after 20 DPI).
- However, more severe vascular changes, mainly haemorrhage and oedema, are reported compare to in the acute form.
Chronic forms
- Low virulence isolates can cause of the disease, clinical signs and lesions are not specific but may persist for several months, giving rise to a range of conditions with symptoms such as skin ulcers and arthritis, delayed growth, emaciation, pneumonia and abortion.
- Characterised by the absence of vascular lesions and low mortality rates, but signs such as delayed growth, emaciation, joint swelling, skin ulcers and lesions associated with secondary bacterial infection.
Laboratory diagnosis
For diagnosis identification of virus done by virus isolation, detection of antigen in smears or section of tissues using FAT and detection of genomic DNA by PCR or real-time PCR. Serological tests pigs surviving natural infection develop antibodies 7-10 days post-infection and can be tested for antibody detection by ELISA, IFAT, IPT.
Vaccine development for ASF
Though vaccination is one of the best control measures for various infectious diseases; development of vaccines against ASFV has been deterred by large gaps in our knowledge of ASFV infection and immunity. There is currently no vaccine available to prevent or control ASF. Various strategies have been used in the search for an effective vaccine for this disease for over 40 years.
| Vaccine Type | Advantages | Disadvantages | Comments |
| Subunit vaccines (recombinant proteins, DNA vaccines, virus vector) | Safety | Requirement for boost likely | Partial protection achieved with recombinant proteins or by DNA vaccination |
| DIVA compatibility | Knowledge of protective antigens required | ||
| Established scale up method | |||
| High containment not required for production | |||
| Inactivated virus
Live attenuated vaccines |
Safety | Mainly stimulates antibody response | Not effective for ASFV |
| Stimulates both cellular and antibody responses | Safety issues relating to adverse post-vaccination reactions and virus persistence | Natural attenuated and gene deleted viruses tested | |
| Single dose may induce long term immunity | Production requires high containment and is virus specific | Optimised combinations of gene deletions required to achieve acceptable levels of safety and efficacy | |
| Knowledge of protective antigens not required | DIVA compatibility more difficult | Cell culture methods for commercial production needed | |
| High efficacy can be achieved |
General approach to control ASF disease
- Early detection of clinical signs suggestive of African Swine Fever (ASF) and prompt reporting to enable diagnosis as soon as ASF enters the country.
- ASF must be contained at the site where it is detected and eradicated as quickly and effectively as possible so that it cannot be reintroduced. •
- Reduce the risk of further spread of African Swine Fever from premises associated with or near the infected premises
- Prior to easing restrictions, conduct risk assessments, and prior to lifting restrictions, conduct surveillance for signs of additional disease
- Comply with existing national laws as well as international trade obligations under the OIE disease control codes.
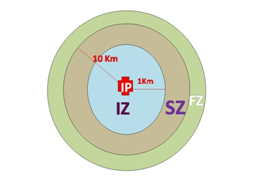
Control strategy
The proposed control strategy divides the pig population of the Country in 3 different subpopulations –
- Infected zone (IZ) – 1 Km radius of infected premises (IP)
- Intermediate/ Surveillance zone (SZ) – 10 Km radius from the infected premises (9 km outside the IZ)
- Disease Free zone/ Non-Infected area (FZ) – Area outside the SZ
Conclusion
ASF is a highly contagious disease that kills 100% of pigs and wild boars, resulting in massive economic losses for pig farmers. There is currently no commercially available vaccine. Pigs that have been affected or come into contact with the virus will be slaughtered and buried deep. The infected sheds and premises must be thoroughly cleaned and disinfected before restocking 4 months later from a known source of healthy farms. The ASF has been reported from neighbouring countries in India’s north eastern states and can enter at any time and persist in our pigs. As a result, one must remain vigilant and alert.
References
- Dixon, L.K., Chapman, D.A.G., Netherton, C.L., Upton, C. (2013). African swine fever virus replication and genomics. Virus Research 173, 3-14.
- Gallardo, M. C., de la Torre Reoyo, A., Fernández-Pinero, J., Iglesias, I., Muñoz, M. J., & Arias, M. L. (2015). African swine fever: a global view of the current challenge. Porcine Health Management, 1(1), 1-14.
- Patil, S. S., Suresh, K. P., Vashist, V., Prajapati, A., Pattnaik, B., & Roy, P. (2020). African swine fever: A permanent threat to Indian pigs. Veterinary World, 13(10),
- Penrith, M.L., Thomson, G.R., Bastos, A.D.S., Phiri, O.C., Lubisi, B.A., Du Plessis, E.C., Macome, F., Pinto, F., Botha, B. and Esterhuysen, J. (2004). An investigation into natural resistance to African swine fever in domestic pigs from an endemic area in southern Africa. Rev Sci Tech, 23(3), 965-77.
| The content of the articles are accurate and true to the best of the author’s knowledge. It is not meant to substitute for diagnosis, prognosis, treatment, prescription, or formal and individualized advice from a veterinary medical professional. Animals exhibiting signs and symptoms of distress should be seen by a veterinarian immediately. |









Be the first to comment