Abstract
Corona viral diseases are seen in animals, birds and human being. α & β corona viruses affects human being (causing diseases like SARS & COVID- 19), whereas gamma corona viruses are causing diseases in birds (Chickens, turkeys, pheasants, quail and partridges). Among these bird diseases, Infectious bronchitis (IB) is the most important disease affecting young birds. IBV is a significant pathogen of commercial meat & egg type birds. Young chicks less than 6 weeks old are mostly affected.
Disease can be identified earlier on the basis of typical clinical signs (Nasal discharge, sneezing, coughing, deformed and misshapened eggs, depression), lesions (exudates in the trachea, nasal passages and sinuses, cloudy air sacs, egg peritonitis, swollen & pale kidneys), but for confirmative diagnosis of disease, virus isolation (chicken embryo inoculation), serology (HI, ELISA), molecular methods (RT PCR) and virus neutralization test (VN) are useful. Biosecurity and immunization of birds is the key to control this disease.
Key words: Corona virus infection, infectious bronchitis (IB) of chickens.
Introduction
- India is an agriculture-based country where farmers mostly engage themselves in agriculture and animal husbandry practices. Poultry industry in India is considered to be most dynamic and the fastest growing industry among the agriculture-based industries. However, during the past few decades, Poultry farming has developed into a vibrant, full-fledged organized commercial industry with the growth rate of 8-10% in egg production and 12-15% in meat production. India occupies 3rd, 4th and 6th rank in egg, layer and broiler production, respectively (Dhama et al., 2014).
- The family Coronaviridae, with a single-stranded, positive-sense RNA genome is a well-known and studied family of viruses in Veterinary medicine. Virtually every pet breed, or companion animal and every wild animal can be said to have dealt with at least one virus from this family in its life.
- Avian Coronavirus belongs to the genus Gamma coronavirus that includes three major species – infectious bronchitis virus (IBV), pheasant Coronavirus (PhCoV), and turkey Coronavirus (TCoV). IBV or IBV-like gamma corona viruses have been found in other avian species such as peafowl, partridge, blue-winged teal, pigeon, guineafowl, and various wild bird species (Bonilauri and Rugna, 2021).
- In poultry, corona virus causes highly contagious, economically important disease affecting the respiratory system in growing birds, reproductive system in hens, with the decrease in production & quality of eggs, also renal systems in some strains. This disease is knowns as Infectious Bronchitis (IB) infection.
- Infectious bronchitis diseases is an acute and highly contagious viral disease caused by Corona virus which bring piles of economic loss in the form of inefficient feed conversion, decreased production, mortality, loss of market value and increased production cost etc.
- ‘All-in /all-out’ operations of rearing along with good biosafety measures forms the basis of prevention, whereas, vaccination forms the backbone of IB control programme. Both live and inactivated conventional vaccines are available. However, vaccination is less successful because of continuous emergence of antigenic variance and less cross protection between the variants. Since the disease continues to emerge with diversity, it is much difficult to control infection as no specific treatment is available. Therefore, early diagnosis and concentrated efforts are necessary to reduce the impact of disease (Priyanka et al., 2019).
Epidemiology
- Numerous coronaviruses, first discovered in domestic poultry in the 1930s, cause respiratory, gastrointestinal, liver and neurologic disease in animals.
- Coronaviruses were first detected in the year 1931 as an acute respiratory infection of chickens called as Infectious Bronchitis virus (IBV) and since then it has been documented in all countries with an intensive poultry industry.
- It has a wide geographical distribution and it was found in regions of Africa, Asia, Australia, Europe, and the United States (Khataby et al., 2016). There are several widely distributed classic and variant IBV genotypes (DeWit et al., 2011). Some IBV genotypes and serotypes are closely related to the vaccine strains while others are variants that are unique to their geographical regions (Bande et al., 2017).
- Generally, IBV serotypes show variations in approximately 20% – 25% in their S1 glycoprotein sequences. However, the variation can sometimes be as high as 50%, which affects the cross-protection toward virus strains. Recently, a S1-gene-based phylogenetic classification of IBV identified 6 different viral genotypes, 32 distinct lineages, and several unassigned recombinants with interlineage origin. Interestingly, the distribution and diversity of these IBV genotypes differ with geographical location (Ennaji et al., 2020).
- In 1940s detected Transmissible Gastroentiritis virus (TGEV).
- Human Coronaviruses were isolated in 1960. Prior to 2002-03 they were believed to cause only mild respiratory illness in humans, but the emergence of severe acute respiratory syndrome virus (SARS), many new ones including MERS have since been discovered with zoonotic potential.
- The number of avian species in which coronaviruses have been detected doubled in last couple of years i.e., Coronaviruses of domestic Fowl, Turkeys & Pheasants (Dave Cavanagh, 2007).
- Several different serotypes can co-circulate in the same area at the same time. Some are found worldwide, but others have more restricted geographical spread. For example, some are found only in Europe; others only in the USA (Intervet International B.V.,2021).
Virus description
- Corona viruses are divided into 4 main groups based on genetic and antigenic characteristics designated as alpha, beta, gamma and delta coronaviruses.
| Alpha coronaviruses | Beta coronaviruses | Gamma coronaviruses (Avian Group) | Delta coronaviruses | |
| TGEV, FIPV, CCV, PRCV, HCoV-229E, RbCoV, FECoV | MHV, BCV, HCoV-OC43
SARS-CoV, MERS-CoV SARS-CoV-2 (COVID-19) |
IBV
TCoV |
Bulbul, Munia & Thrush | |
(Quinn et al.,2002)
- IBV comes under gamma Coronavirus
- The Infectious Bronchitis Virus is a coronavirus in the order Nidovirales.
- It is large, pleomorphic, enveloped virus contain a single molecule of linear, positive-sense, single stranded RNA genome of approximatively 27 kb (Legnardi et al.,2020).
- Replicate in cytoplasm.
- Family – Coronaviridae.
- The virus contains “Club-shaped” glycoprotein peplomers projecting from the envelope impart a “Crown” like appearance to virus (Quinn et al.,2002).
- The virions are sensitive to heat, lipid solvents, formaldehyde, oxidizing agents and non-ionic detergents (Quinn et al., 2002).
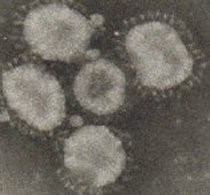
- The virus can survive at pH 6 to 7.3 (Bande et al., 2017).
- It can be inactivated after 15 minutes at 56°C and after 90 minutes at 45°C.
- IBV has an enormous capacity to change both by spontaneous mutations and by genetic recombination (Cavanagh & Gelb, 2008).
Serotypes
- The virus has grouped into 20 or more serotypes.
- The best-known serotypes include: Massachusetts, Connecticut (affinity for respiratory tract), Arkansas-99, Holland, JMK, Florida along with numerous others, distributed round the globe (Vegad et al., 2018).
- Other serotypes Holte, Gray and Australian-T associated with nephrosis (Dhama et al., 2014).
Susceptibility
- The IB virus causes infection mainly in chickens and is a significant pathogen of commercial meat and egg type birds (Dave Cavanagh, 2007).
- IB affects chickens of all ages (Britton & Cavanagh, 2007 and Cavanagh et al., 2002).
- However, it is severe in young chicks less than 6 weeks old.
- Other species such as turkeys, pheasants, quail and partridges can also get infection.
Morbidity & Mortality rate
- Morbidity may approach 100% and Mortality may be high as 25% or more in chicken less than 6 weeks of age (Vegad et al., 2015). The virus shed from the respiratory tract for a few weeks after infection, may be recovered over a period of weeks from the faeces and from eggs of infected birds. Infection may persist in the digestive tract of individual birds (Quinn et al., 2002).
- The mortality rate is dependent on the age of the chickens when infected, and the presence of secondary invading organisms such as coli. (P. J. Quinn, et.al.,2001).
Transmission
- The IB virus spreads horizontally by aerosol transmission (sneezing), through contaminated organic material, drinking water and equipments.
- Surface contamination of eggs with the IB virus is a possible way by which the virus can be spread in Hatcheries or Egg Packing Stations.
- Vertical transmission has no much importance (Quinn, al.,2001).
Incubation period
- The incubation period is relatively short i.e., 18- 48 hours (Quinn, al.,2001).
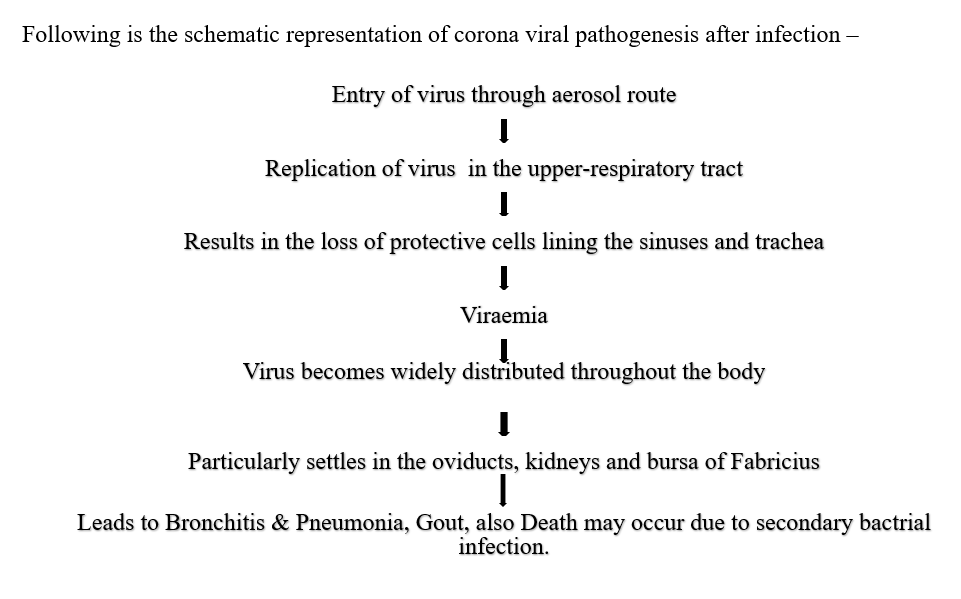
Pathogenesis
- It depends on the age of the bird, host immune status, and virulence of the virus.
- As age increases, chickens become more resistant.
Following is the schematic representation of corona viral pathogenesis after infection
Clinical sings
- Young chickens are depressed and huddle under the heat source.
- Respiratory signs: It includes Gasping, coughing, sneezing, tracheal rales, nasal discharge and watery eyes.
- Birds in lay have a marked drop in egg production and an increased number of poor-quality eggs i.e., thin, wrinkled, roughened eggs may be produced.
- The external and internal quality of the eggs: The external & internal qualities of eggs may be affected, resulting in misshapen or soft‐shelled eggs with watery albumin, loss of pigment colour of eggshells, Also, the hatchability rate of the eggs may be affected.
- When the kidneys are affected, increased water intake, depression, scouring and wet litter are commonly observed.
Post-mortem lesions
- Serous, catarrhal, or caseous exudates are found in the trachea, nasal passages and sinuses.
- Cloudy air sacs, may contain yellow caseous exudates.
- Caseous plug may be found in the trachea.
- Pneumonia may be seen.
- Swollen, pale kidneys, with distended tubules and ureters containing urate crystals in nephro-pathogenic cases.
- Fluid yolk material may be found in the abdomen of birds in production (egg peritonitis).
- Degeneration of the ovary and swollen oviducts can be seen.
Sample collected
- In live birds: tracheal swab in 1st week & cloacal swab after one week.
- In dead birds: trachea & lungs for respiratory form, kidney, testes, oviduct, caecal tonsils or proventriculus in other forms.
Diagnosis
- History & Clinical signs: Nasal discharge, sneezing, coughing, deformed and misshapen eggs, depression like symptoms are seen.
- Virus isolation and identification: Specific pathogen free chicken embryos or tracheal organ cultures (TOCs) from embryos may be used for virus isolation. Following inoculation of the allantoic cavity, IBV produces embryo stunting, curling, clubbing of the down, or urate deposits in the mesonephros of the kidney, usually within three serial passages.
- RT-PCR: (Reverse-transcription polymerase chain reaction). To identify the spike (S) glycoprotein genotype of IBV field strains gives sure diagnosis.
- Serology: Haemagglutination inhibition (HI) tests to determine serotype and enzyme-linked immunosorbent assays (ELISA) for general monitoring.
- Supplementary tests: It include electron microscopy, the use of monoclonal antibodies, virus neutralisation (VN), immunohistochemical or immunofluorescence tests, and immunisation-challenge trials in chickens.
Differential diagnosis
Infectious bronchitis may resemble other acute respiratory diseases such as Newcastle disease (ND), Avian influenza and Infectious laryngotracheitis, and can be differentiated as:
- Newcastle disease: It is caused by Paramyxovirus type 1 having different pathotypes (Velogenic Viscerotropic or Neurotropic, Mesogeneic, Lentogenic) produces greenish diarrhea, torticolis, paralysis of one side of leg and wing and characteristic pinpoint hemorrhages on tip of proventriculus and on caecal tonsil. The velogenic strain having higher mortality than IB.
- Avian influenza: It is caused by Orthomyxovirus type a characterized by cyanosis of comb, wattle & shank region causing100% mortality of affected flock in highly pathogenic strains of avian influenza (HPAI) infection while Low pathogenic strains of avian influenza (LPAI) produce mild to moderate respiratory disease with low mortality and thus, may resemble IB.
- Infectious Laryngotracheitis: It is caused by Gallid herpes virus type 1.
Respiratory signs may be more severe than IB like “Pump Handle Respiration”, and open mouth breathing and characteristic hemorrhagic tracheitis and caseous plaque in trachea and intranuclear inclusion bodies in tracheal epithelium may be evident during microscopic examination. - Infectious coryza: It is caused by Haemophilus paragallinarum characterized by edematous swelling of head which is absent in IB.
Prevention
A) Vaccination
- Live vaccines
Ma5 vaccine (Massachusetts strains), IB 4/91 vaccine & IB Primo QX vaccine in Sphereon format (Variant strain as stated in MSD, 2021).
Route of administration: Given by oral or aerosol route at 14 days of age & again at 4 weeks of age (Quinn, et.al.).
- Inactivated vaccines
B) Biosecurity measures
- Basic management practices such as limited controlled site access, separate footwear and equipment for each site/house, and footbaths at the entrance to sites/houses all minimize the risk of introducing the Infectious Bronchitis virus (IBV).
- Hygienic measures: Follow all biosecurity measures on your farm. A structured approach – It includes Wet clean, dry clean & disinfection.
C) Quarantine: It is necessary to quarantine new chickens before adding them to the flock.
D) All in – All out System can be preferred.
Conclusions
- Corona viral diseases are not only the human diseases but also the animals, birds are affected by it.
- There is no evidence that the human corona virus can affect the chicken and vice-versa.
Acknowledgment
Authors are thankful to the Associate Dean of COVAS, Parbhani, for providing facilities required.
References
- Amalendu chakrabarthi (2017), A Textbook of Preventive Veterinary Medicine, 6th Edition, Kalyani publishers, pp 605-607.
- Bande, F., Arshad, S. S., Omar, A. R., Hair-Bejo, M., Mahmuda, A., & Nair, V. (2017). Global distributions and strain diversity of avian infectious bronchitis virus: a review. Animal health research reviews, 18(1), 70-83.
- Britton, P. (2007). Avian coronavirus diseases and infectious bronchitis vaccine development. Coronaviruses: molecular and cellular biology, 161-181.
- Cavanagh D, 2002, Coronaviruses from pheasants (Phasianus colchicus) are genetically closely related to coronaviruses of domestic fowl (infectious bronchitis virus) and turkeys, 81-93
- Cook, J.K.A. (1983), Veterinary Record, 112, 104 ‐105.
- Dave Cavanagh (2005) Coronaviruses in poultry and other birds, Avian Pathology, 34:6, 439-448 (DOI: 10.1080/03079450500367682)
- De Wit, J. J., Cook, J. K., & Van der Heijden, H. M. (2011). Infectious bronchitis virus variants: a review of the history, current situation and control measures. Avian pathology, 40(3), 223-235.
- Dhama, K., Singh, S. D., Barathidasan, R., Desingu, P. A., Chakraborty, S., Tiwari, R., & Kumar, M. A. (2014). Emergence of Avian Infectious Bronchitis Virus and its variants need better diagnosis, prevention and control strategies: a global perspective. Pakistan journal of biological sciences: PJBS, 17(6), 751-767.
- Epub 2007 Feb 13, Vet Res.; 38(2):281-97. (doi: 10.1051/vetres:2006055).
- Ennaji, Y., Khataby, K., & Ennaji, M. M. (2020). Infectious bronchitis virus in poultry: Molecular epidemiology and factors leading to the emergence and reemergence of novel strains of infectious bronchitis virus. In Emerging and Reemerging Viral Pathogens(pp. 31-44). Academic Press.
- Khataby, K., Fellahi, S., Loutfi, C., & Mustapha, E. M. (2016). Avian infectious bronchitis virus in Africa: a review. Veterinary Quarterly, 36(2), 71-75.
- Legnardi, M., Tucciarone, C. M., Franzo, G., & Cecchinato, M. (2020). Infectious bronchitis virus evolution, diagnosis and control. Veterinary Sciences, 7(2), 79.
- Ganti A. Shastry (2001), Veterinary Pathology, 7th Edition, CBS publishers, pp 663- 665.
- http://www.poultrydvm.com/condition/infectious-bronchitis
- MSD Animal Health, Intervet International B.V.,2021
- J. Quinn, B.K. Markey, W.J. Donnelly and F.C. Leonard (2001), Veterinary Microbiology and Microbial Disease, 2nd Edition, Wiley Blackwell, pp 413-421.
- Vegad, J. L. (2018). Poultry diseases: a guide for farmers and poultry professionals (second revised and enlarge edition) International Book Distributing Company.
| The content of the articles are accurate and true to the best of the author’s knowledge. It is not meant to substitute for diagnosis, prognosis, treatment, prescription, or formal and individualized advice from a veterinary medical professional. Animals exhibiting signs and symptoms of distress should be seen by a veterinarian immediately. |






Be the first to comment