Subclinical mastitis (SCM) is subtle and more difficult to detect as the affected cow appears healthy, the udder does not show any signs of inflammation and the milk appears normal. Staphylococcus aureus (S. aureus) and Streptococcus agalactiae (S.agalactiae) are the leading oganisms to cause infection of other cows (Bramley et al., 1996 and Riffon et al., 2001). The major pathogens responsible for bovine mastitis can be classified as contagious pathogens (S. aureus, S. agalactiae) and environmental pathogens {Escherichia coli (E. coli), Streptococcus dysgalactiae (S. dysgalactiae) and Streptococcus uberis (S. uberis)}.
1. Early diagnosis of Subclinical mastitis (SCM) by California Mastitis Test
For Subclinical mastitis (SCM) early diagnosis by California mastitis test (CMT) Iqbal et al. (2006) analyzed 352 cases for subclinical mastitis by CMT. Statistical analysis proved effectiveness and sensitivity of CMT at both farm and laboratory levels.
California Mastitis Test reagent
Composition
- Sodium hydroxide 1.50 g
- Teepol 0.50 ml
- Bromothymol blue 0.01g
- DW upto 100.0 ml
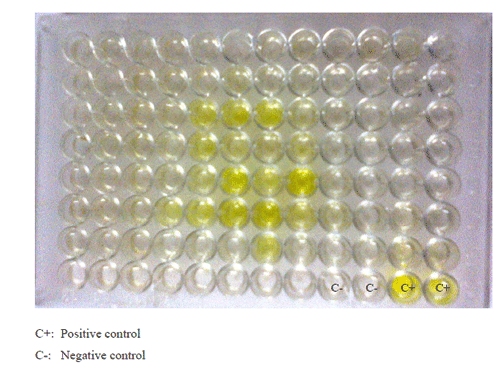
California Mastitis Test
The test procedure recommended by Rajani and Sharma (1965) was employed.The test was performed by mixing 50μl of CMT reagents with equal volume of milk samples on a clean, grease free microscope slide The results were interpreted based on the presence of precipitate or gel formation.
A study was carried out to sequentially monitor the sub-clinical cases of bovine mastitis due to major bacterial pathogens such as S. aureus, E. coli, S. agalactiae,S. dysgalactiae and S.uberis. milk samples sequentially collected from 1st to 88thday. First five collections were made at a weekly interval (i.e. 1st, 7th, 14th, 21st and 28th days) and later, fortnightly for two months (i.e. 43rd, 58th, 73rd and 88th days). For the purpose of studying the sequential prevalence of SCM, 225 milk samples from 25 cows were included in the study.
Prevalence of SCM in dairy cows by CMT
The overall prevalence of SCM was 86.66 per cent (195/225) by CMT. The CMT values were recorded as 0, +, ++ and +++. The highest prevalence of SCM was recorded on days 1st and 43rd (100 per cent, 25/25), followed by day 58th (96 per cent, 24/25), days 21st and 88th (92 per cent, 46/50), day 7th (88 per cent, 22/25) and day 73rd. (84 per cent, 21/25). The least prevalence of SCM (64 per cent, 34/50) was seen on days 14th and 28th Students t-test was performed on results of CMT with milk samples from all 9 collections. No significant difference was observed between any of the collections in CMT (P>0.05).
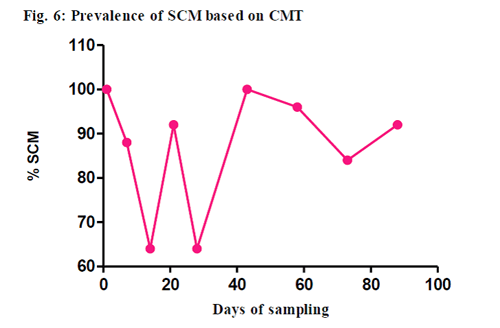
The CMT could be useful as a regular mastitis screening test in dairy herd monitoring programs even by less trained dairyman as supported by Sergeant et al.(2001). In our study, based on CMT, the prevalence of SCM was 86.66 % .This was in accordance with Moatamedi (2006) who also reported 83.3% prevalence and in contrast to various others authors who reported relatively lesser prevalence ranging from 19.18 % to 65.73 % (Nagahata et al., 1987; Shem et al., 2001; Anju, 2007).
2. Early diagnosis of Subclinical mastitis (SCM) by by Somatic Cell Count (SCC)
Streptococcus agalactiae, S. dysgalactiae and S. uberis are Gram-positive bacterial pathogens that affect cows in dairy herds. These are considered as the major causes of economic losses of dairy producers without a control rogram. Subclinically infected cows are intermittent shedders of organisms and may cycle through low and high shedding patterns during lactation , subclinically infected glands due to the presence of very low number of pathogens,may hinder diagnosis. The presence of leukocytes in milk samples from cases of clinical mastitis and in milk samples with high somatic cell counts may also potentially inhibit growth of bacteria (Phuektes et al., 2001). The somatic cells counting (SCC) is a tool to detect sub clinical and assistant infection in the evaluation of milk quality. The main purpose of this research was to evaluate the degree of the sub clinical mastitis of the dairy herd. The high value of SCC suggests that the quality of the milk produced could be affected and that some cows of the herd can be with sub clinical mastitis,The SCC level considered normal is under 200.000 cells/ml of milk, although it may be less in the first lactation. Milk samples sequentially collected from 1st to 88thday. First five collections were made at a weekly interval (i.e. 1st, 7th, 14th, 21st and 28th days) and later, fortnightly for two months (i.e. 43rd, 58th, 73rd and 88th days). For the purpose of studying the sequential prevalence of SCM, 225 milk samples from 25 cows were included in the study.
SCM BY SCC: Nucleocounter (M/s. Chemo Metec, Denmark), Lysis Buffer was procured from M/s. Chemo Metec. Denmark
Somatic Cell Count (SCC)
Fresh milk samples were used for SCC estimation. Somatic Cell Count was estimated using Nucleocounter (Chemo Metec, Denmark) following the instructions given by the manufacturer. Five hundered microlitre of milk samples was mixed with equal quantity of the lysis buffer supplied by the manufacturer. The mixture was mixed gently to lyse the cells and was aspirated into the cassette by pressing the piston. The cassette was then inserted into the Nucleocounter and the SCC values were recorded. The SCC value of more than 5,00,000 cells/ml of milk was taken as criteria to declare the milk /animals as subclinical mastitic
Statistical Analysis
The data generated in the study was statistically analyzed by student ‘t’ test and One way, two way ANOVA at p≤0.05, post tests Bonferroni’s and Dunnett’s by using statistical software to arrive at the conclusion.
Prevalence of SCM by SCC
The SCC revealed an overall prevalence of SCM at 73.33 per cent (165/225) with 5 lakh as cut-off. The high prevalence of SCM was noticed on days 1st and 43rd (100 per cent, 25/25) with decreasing order on 88th (96.00 per cent, 24/25), 21st (92.00 per cent, 23/25), 7th (76 per cent, 19/25), 14th and 28th (56 per cent, 28/50), 58th (48 per cent, 12/25) and least on day 73rd (32 per cent, 8/25)
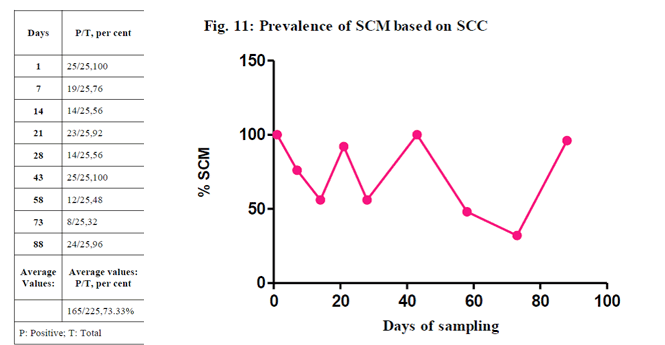
(Narayana and Iya,1954). Accordingly, the factors that influence the SCC are the differences among the herds, among the order of partum, among lactation phases and among breed. The results presented in Table 1 agree with those registered in the literature, but, there are still factors related to the sanitary management that may directly influence on SCC, accordingly, authors which have pointed out that an increase in SCC is related to the increase of infection prevalence.
A broad program of mastitis control based on the prevention may cause a decrease in loses associated to mastitis, improve of milk quality and to increase the production. The magnitude of the answer in SCC to the presence of mainly pathogens Staphylococcus aureus, Streptococcus agalactiae, Streptococcus spp and coliforms is variable for each cow and it would be impossible to identify different strains, basing only on SCC. Thus, the milk evaluation could be impaired. It is ecessary further researches about the validity of SCC as a tool in the prevention of cases of mastitis and evaluation of milk quality.
3. Early diagnosis of Subclinical mastitis (SCM) by EC (Electrical conductivity)
Electrical conductivity of quarter milk was evaluated for accuracy in detection of subclinical mastitis compared with that of bacteriological culture and SCC of sampled quarters. Because of the low prevalence of mastitis, were sampled. All sampled were. The system identified correctly 18 out of 23 subclinical and 521 out of 555 healthy samples prevalence was about 1%. Predictive value of a positive test (35%) and the predictive value of a negative test (99%) were calculated, as well as sensitivity (25%) and specificity (99%), after extrapolation of the total number of samples. Because of repeated measurements, sensitivity may be underestimated. When samples were defined by repeated positivity within 14 d, predictive value positive increased to 48%. The EC did not detected subclinical mastitis very accurately because of suboptimal test characteristics Kasikci et al. (2012) studied a total of 386 milk samples collected from quarters of 188 cows at 10 different farms. Of the samples, 258 (66.85 per cent) were CMT (+), 85 (22.02 per cent) were CMT (++) and 43 (11.13 per cent) were CMT (+++). The mean EC and SCC were 25.71, 28.02 and 29.63 m S/cm and 249453, 1167058 and 2108139 cells, respectively, according to CMT results. The total viable bacterial counts ranged from 3.4771 to 6.9395, from 3.4771 to 7.3617 and from 4.7782 to 7.5315 log CFU/ml for different CMT values, respectively. It was concluded that EC showed correlation with the CMT and SCC in the detection of subclinical mastitis, its reliability would further increase when used together with the other diagnostic tests.
Electrical Conductivity: Milk Checker (Eisai Co. Ltd. and Orient Instruments Ltd., Tokoyo, Japan) Potassium chloride-procured from M/s. Sisco Research Laboratories Pvt. Ltd., Mumbai.
Prevalence of SCM in dairy cows by Electric conductivity
Overall, the prevalence of SCM was (81.77 per cent, 184/225) by EC. With high prevalence on day 28th (96 per cent, 24/25), followed by day 21st(92 per cent, 23/25), 43rd (92 per cent, 23/25), 58th (88 per cent, 21/25 ), 1st (84 per cent, 20/25), 7th, 14th(80 per cent, 20/25) and day 73rd (72 per cent, 18/25) and the least prevalence on day 88th (52 per cent,13/25). Students t-test was performed on per cent prevalence of SCM based on EC for all the milk samples collected on day one to day 88. No significant difference (p>0.05) was observed in the prevalence of SCM.
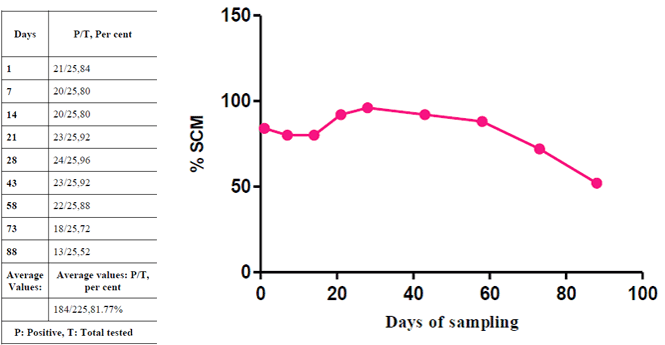
In the present study, EC of more than 6.5 mS/cm was considered as positive for the detection of SCM in diary cows as per Swarup et al.(1989). Based on this criteria, the prevalence of SCM was 80.88% (182/225) which is in accordance with the work of Nithinprabhu (2013) (80.37%). However, variable SCM prevalence based on EC has been reported by Bansal, et al. (2005) (73%), Anju (2007).Spakauskas et al. (2006) observed that the EC based SCM was at 78.30% (794/1014). However, even though in cases of pronounced reaction with tests, EC has tendency to increase, without always correlating with SCC and CMT. Discrepancy of the results may be explained by the fact that EC depends on the number of ions, the number of which in milk increases much later than SCC.
4. Early diagnosis of Subclinical mastitis (SCM) by NAGase assay (NAGase test)
For diagnosis of prevalence of SCM, milk samples were analyzed by Emanuelson et al. (1987) for somatic cell counts, ATP, NAGase, bovine serum albumin, antitrypsin, conductivity and bacteriological findings. The diagnostic tests were compared with respect to their ability to predict the infection status. Predictive ability was highest for ATP, SCC and NAGase. Comparisons using ratios from different quarters gave no better prediction than absolute values..Ball and Greer (1991) showed the use of NAGase test for detecting subclinical mastitis from 20 farms. Milk samples were considered to be mastitic if they had milk cell count above 400,000 cells/ml, and the NAGase test results were graded accordingly. The test gave an average of 16.6 per cent false positives and 2.0 per cent false negatives per herd. It was concluded that the NAGase test could be used as a rapid screening method for selecting milk samples.
NAGase assay: To study the prevalence of subclinical mastitis, a total of 250 bovine composite milk samples were collected from an organized farm. The relevant information with respect to age, stage of lactation and milk yield were also noted. Approximately, 10 ml of milk was collected in sterile tubes following strict aseptic measures and was immediately transported to laboratory on ice. To sequentially monitor subclinical mastitis, milk samples were collected from all twenty five animals on days 1, 7, 14, 21,28 and then fortnightly for two months. The collected milk samples were screened for SCM by EC, CMT, SCC, NAGase, LPB-ELISA and M-PCR.
Screening for subclinical mastitis: All the 225 milk samples collected from an organized farm on days 1, 7, 14, 21,28, and fortnightly for two months were subjected, N-acetyl Beta-Dglucosaminidase (NAGase) estimation and for detecting SCM.
Material and methods
NAGase
- N-acetyl beta D glycosaminide (substrate) was obtained from M/s. Sigma Aldrich Co. Pvt. Ltd., USA.
- Glycine–procured from M/s .Hi-Media Laboratories, Mumbai.
- Sodium deoxycholate –procured from M/s.Hi-Media Laboratories, Mumbai.
The following chemicals were procured from M/s. SD Fine Chemicals Pvt. Ltd. and M/s. Sisco Research Laboratories Pvt. Ltd., Mumbai and used in this study.
- Sodium citrate
- Citric acid
- Chloroform
N- acetyl- Beta- D- Glucosaminidase (NAGase) estimation
NAGase enzyme was estimated as per the method described by Kitchen, (1976). Initially, 0.2 ml milk sample was mixed with 0.3 ml of the substrate in a small polypropylene tube.
- The tubes were incubated for 15 min. at 50 o C in a water bath.
- Reaction was stopped by adding 1 ml of 1 M glycine containing 1% deoxycholate (pH adjusted to 10 with sodium hydroxide).
- One ml of chloroform was added and shaken vigorously for 5 sec.
- Tubes were centrifuged at 2000 g for 10 min.
- Top aqueous layer was removed with a pasture pipette.
- Absorbance was recorded at 405 nm.
The OD value 0.1 at 405nm was taken as positive index of sub-clinical mastitis. The data generated in the study was statistically analyzed by student ‘t’ test and One way, two way ANOVA at p≤0.05, post tests Bonferroni’s and Dunnett’s by using statistical software to arrive at the conclusion
Prevalence of SCM by NAGase test
The overall prevalence of SCM was 77.33 per cent, (174/225) by NAGase test. The high prevalence (100 per cent, 25/25) of SCM was observed on day 1st. This was followed by days 14th (84 per cent, 21/25), 58th (80 per cent, 20/25), 88th (76 per cent, 19/25), 73rd (72 per cent, 18/25), 21st and 43rd (68 per cent, 34/50) and day 7th (48 per cent, 12/13). The low prevalence of (44 per cent, 11/25) SCM was observed on day 28th
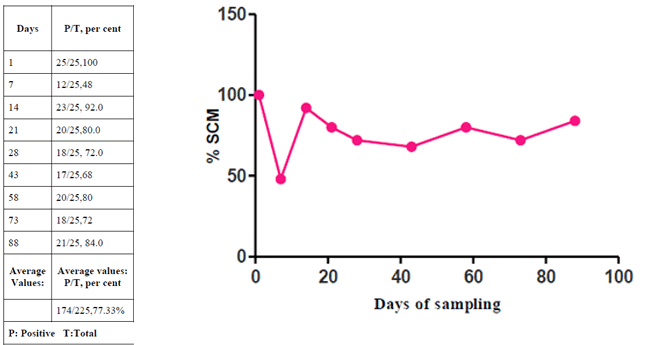
The automated method based on biomarker such as NAGase in the diagnostic laboratory. Biomarkers are chemical substance which are released very early during an infection or inflammation. In present study, the high prevalence of SCM by NAGase at 77.33 % was similar to the observation of Kitchen et al., 1984 (74%) and Bansal et al., 2005 (73%). Further, Kitchen (1976) considered NAGase test to be most reliable and sensitive,
5. Early diagnosis of Subclinical mastitis (SCM) by Milk Yeild (MY)
The milk production in India increased by 3 million MT per annum from 1992 to 2007 (White Paper Draft Committee, 2012). To maintain the status as the global leader in milk production, it needs to increase milk production at 5 million MT per annum in next fifteen years. the causes of decreasing the milk production need to be controlled amongst which the bovine mastitis is the major one. Mastitis occurs worldwide among dairy animals and it has been described to have an extreme economic impact (Al-Majali et al., 2008). Subclinical mastitis (SCM) is subtle and more difficult to detect as the affected cow appears healthy, the udder does not show any signs of inflammation and the milk appears normal. Rabbani and Samad, (2010) showed negative correlation between SCM and milk yield, the prevalence of SCM at high, medium and low level of milk production in HFC. found significantly (p<0.01) correlated with milk production
Collection of milk samples
To study the prevalence of subclinical mastitis, a total of 250 bovine composite milk samples were collected from an organized farm. The relevant information with respect to age, stage of lactation and milk yield were also noted. Approximately, 10 ml of milk was collected in sterile tubes following strict aseptic measures and was immediately transported to laboratory on ice. To sequentially monitor subclinical mastitis, milk samples were collected from all twenty five animals on days 1, 7, 14, 21, 28 and then fortnightly for two months.
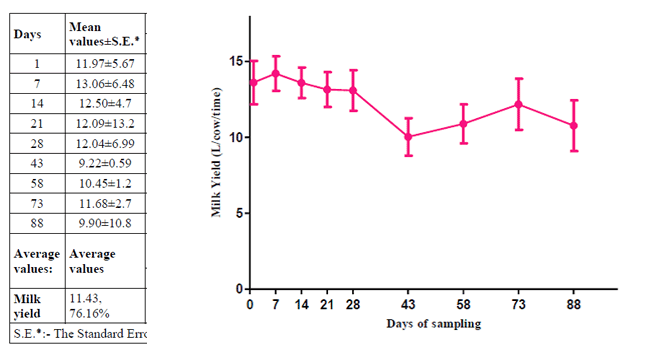
An overall 73.61 per cent cows were affected with SCM, which decreased milk production by 23.94 per cent. Average milk loss was 2159.2 litres/cow/lactation with a milk yield 6868.1 litres /cow/lactation for milk samples collected in the evening, for 9 times at a a weekly interval and then at fifteen days interval from 25 Holstein Friesian cows, from an organized farm
Intramammary infection is the primary reason for increased SCC for all types of mastitis. A negative correlation exists between SCC and milk yield (Burvenich et al.,2003), indicating that a great part of the mammary epithelium may be damaged by the invading bacteria and / or the phagocytes that were sent out by the defence system of the host. Decreased or lost milk production is an important aspect of CM.(clinical mastitis) SCM has an important economic effect on dairy industry. Blosser (1979) stated that 70% of the total economic losses resulting in decreased milk yield were attributed to SCM.
In our study, an overall 76.1 % cows were affected with SCM, which decreased the milk production by 23.92 %. Average milk production was 6868.1 liters/cow/lactation with milk loss of 2159.2 litres /cow/lactation for milk sampling in evening, once a day.Lucey and Rowlands (1984) stated that cows with SCM, indicated by SCC of 350,000-450,000 cells /ml may lose up to 300 litres of milk per lactation.
As per our study, the milk loss calculated (23.94%) was in accordance with the work of Islam, et al. (2011) wherein the prevalence of SCM in crossbred were 28.57% ,35.48%, 40.00%, and 42.85%,with >1-2 litre; >2-5litre; >5-10 and >10 litre milk production per day. Even Jansen (1990, 1991) reported that losses in milk production attributable to mastitis were reviewed with losses at the quarter level being found to range from 9% to 43.3% of potential milk production in organised dairy farms.
Conflict of interest: Authors would hereby like to declare that there is no conflict of interests that could possibly arise.
Collected from: GADDI, R., 2017. Sequential monitoring of sub-clinical cases of bovine mastitis due to major bacterial pathogens by molecular and biomarker based approach. PhD Thesis, Karnataka Veterinary, Animal and Fisheries Sciences University, Bidar,India. Presently working as CVO. VD. Dodwad, Bailhongal, Belgavi, Karnataka, Karnataka.
References:
- ANJU CHAHAR., 2007. Studies on comparative evaluation of various screening tests for detection of sub-clinical mastitis in cows. Intas Polivet, 8 (1):208-211
- BRAMLEY, A. J., KING, J. S. and HIGGS, T. M., 1979 .The isolation of Streptococcusuberis from cows in two dairy herds, British Vet. J., 135:262-270
- IQBAL, M., AMJED, M., KHAN, M. A., QURESHI, M.S. and SADIQUE, U., 2006.
- Comparative efficiency of some indirect diagnostic tests for the detection of subclinical mastitis in cows and buffaloes. Pakistan Vet. J., 26 (2):73-79
- MOATAMEDI. H., SEYFIABAD SHAPOURI, M., GHORBANPOOR, M.,JAMSHIDIAN, M. and GOORANINEJAD, S., 2006. A polymerase chainreaction based study on the sub-clinical mastitis caused by Streptococcusagalactiae, S . dysgalactiae, and S.uberis in Cattle in Ahvaz .Iranian J. Vet. Res.,University of Shiraz.,8(3) : 20.
- RAJANI, H.B. and SHARMA, V.K., 1969. California mastitis test. Ind.Vet. J. 46: 749-52
- RIFFON, R., SAYASITH, K., KHALIL, H., DUBRENIL, P., DROLET, M. and LAGACE’, J., 2001. Development of a rapid and sensitive test for identification of major pathogens in bovine mastitis by PCR. J. Clin. Microbiol., 39:2584-2589.
- SEARGENT, J. M., LESLIE, K. E., SHIRLEY, J. E., PULKRABEK, B. J. and LIM, G. H.M., 2001. Sensitivity, specificity of Somatic cell count and CMT for identifying intramammary infection in early lactation. J. Dairy Sci., 84 : 2018.
- SHEM, M. N., MOSHA, F.A., MACHANGU, R., KAMBARAGE, D. and FUJIHARA, T., 2002. Bovine Mastitis in Zebu and crossbred cattle under the extensive management system in Tanzania. Asian- Aust.J.Anim.Sci.,15(5):751-756 .
- NARAYANA, T. and IYA,K. K., 1954.Studies on bovine mastitis. Incidence of mastitis in cows and buffaloes .Indian.J.Dairy.Sci.,6.
- PHUKTES, P., BROWNING, G., ANDERSON, G. and MANSELL, P., 2001. Multiplex polymerase chain reaction as a mastitis screening test for Staphylococcus aureus, Streptococcus agalactiae ,Streptococcus dysgalactiae and streptococcus uberis in bulk milk samples .J.Dairy Res.;70(2):149-55
- ANJU CHAHAR., 2007. Studies on comparative evaluation of various screening tests for detection of sub-clinical mastitis in cows. Intas Polivet, 8 (1):208-211.
- BANSAL.B. K., HAMANN, J., NILSTH. G. and SINGH K.B., 2005. Variation in the composition of selected milk fraction samples from healthy and mastitis quarters and its significance for mastitis diagnosis. J. Dairy Research, 72:144-152
- KASIKCI, G., CETIN, O., BINGOL,E.B. and GUNDUZ, M.C., 2012. Relations between electrical conductivity, somatic cell count, California mastitis test and some quality parameters in the diagnosis of sub-clinical mastitis in dairy cows .Turk .J.Vet.Anim.Sci.,36(1):49-55 .
- NITHINPRABHU. K., ISLOOR,S., HEGDE, R., RATHNAMMA, D., VEEREGOWDA, B.M., NARASIMHA MURTHY, H. N., SHOME, R. and SURYANARAYANA,V.V.S., 2013. Development of polymerase chain reaction for detection of predominant streptococcal isolates causing subclinical bovine mastitis. Indian J. Biotechnol.,12: 208-212 .
- ŠPAKAUSKAS, V., KLIMIENĖ, I. and MATUSEVIČIUS, A., 2006. A comparison of indirect methods for diagnosis of subclinical mastitis in lactating dairy cows. Veterinarski Arhiv., 76 (2): 01-109
- SWARUP, D., KUMAR,P.N. and SINGH,R., 1989. Electrical conductivity of milk for the detection of mastitis. Indian J.Anim.Sci., 59 : 1227-1229.
- BALL, H.J. and GREER, D., 1991. N-acetyl-beta-D-glucosaminidase test for screening milk samples for sub-clinical mastitis. J. Dairy Sci.,85 (5):1133-40.
- BANSAL.B. K., HAMANN, J., NILSTH. G. and SINGH K.B., 2005. Variation in the composition of selected milk fraction samples from healthy and mastitis quarters and its significance for mastitis diagnosis. J. Dairy Research, 72:144-152
- EMANUELSON, U., OLSSON T., HOLMBERG, O., HAGELTORN, M., MATTILA, T., NELSON L. and ASTROM G., 1987.Comparison of some screening test for detecting mastitis. J.Dairy Sci.,70: 880-887 .
- KITCHEN, B. J., 1976. Enzymatic methods for estimation of the somatic cell count in bovine milk. I. Development of assay techniques and a study of their usefulness in evaluating the somatic cell count in milk. J. Dairy Res., 43:251-258.
- KITCHEN, B.J., MIDDLETON, G., and SALMON,M., 1978. Bovine milk N-acetylbeta- D-glucosaminidase and its significance in the detection of abnormal udder secretions .J. Dairy Res., 45(1):15-20 .
- KITCHEN, B. J., 1981. Review of the progress of dairy science : Bovine mastitis: milk compositional changes and related diagnostic tests. J. Dairy Res., 48 : 167-188.
- KITCHEN, B.J, KWEE, W.S, MIDDLETON, G. and ANDREWSRJ., 1984a. Relationship between the level of N-acetyl-beta-D-glucosaminidase (NAGase) in bovine milk and the presence of mastitis pathogens. J.Dairy Res., 51(1):11-16.
- KITCHEN, B.J., MIDDLETON, G., KWEE,W.S. and ANDREWS, R.J., 1984b. Nacetyl- beta-D-glucosaminidase (NAGase) levels in bulk herd milk. J.Dairy Res.,51(2):227-232.
- AL-MAJALI, A. M., Al-QUDAHK. M., Al-TARAZI.Y. H. and Al-RAWSHDEH.O. F., Risk factors associated with camel brucellosis in Jordan. Trop. Anim.Health Prod., 40: 193-200.
- BLOSSER, T. H., 1979. Economics losses from and the national research program on mastitis in the United States. J. Dairy Sci., 62(1) : 119-27
- BURVENICH, C., MERRIS,V.V. MERSZAD, J., ARACELI,D.F. and CHATEAU,L., Severity of E.coli mastitis is mainly determined by cow factors. Vet. Res.,34:521-564
- ISLAM, M. Z. M.A., M.S., ISLAM, M.A. and ISLAM, M.T, 2011, Prevalence of Sub-clinical Mastitis in dairy cows in selected areas of Bangladesh. Bangla. J. Vet. Med., 9(1):73-78 .
- JENSEN, N. and KNUDSEN, K., 1990. Sensitivity ,Specificity and Predictive values of bacteriological tests for diagnosing subclinical infection mastitis. Proc International Mastitis Symposium, pp 46-51.
- JENSEN, N.E. and KUNDSEN, K., 1991, Inter-.quarter comparison of markers of Subclinicalmastitis: Somatic Cell Count ,Electrical Conducrivity, N-acetyl-Dglucosaminidase and antitrypsin. J.Dairy Res., 58:389-399
- RABBANI, A. F. M. G. and SAMAD, M. A., 2010. Host determinants based comparative prevalence of subclinical mastitis in lactating Holestein-Friesian cross cows and Red Chittagong cows in Bangladesh. Bangl. J. Vet. Med., 8(1):17-21.
- WHITE PAPER DOCUMENT, 2012. 1st White Paper Draft Committee, 2012. IAI VISION 2020-1st White Paper Document for Indian Dairy Industry. Dairy Planner, Vol. 8-Issue 4, 2012
|
The content of the articles are accurate and true to the best of the author’s knowledge. It is not meant to substitute for diagnosis, prognosis, treatment, prescription, or formal and individualized advice from a veterinary medical professional. Animals exhibiting signs and symptoms of distress should be seen by a veterinarian immediately. |




Nice article, I’m very proud of u sir. All the best.
Wonderful article sir …it’s one of best and easy method to detect mastitis in field level ..
Very useful in field
Good article on mastitis .
Very use full at field level .
Well complied information… excellent
FIELD ORIENTED RESEARCH, VERY USEFUL AND INFORMATIVE.
Very useful, timely and well written. Congratulations and keep up the spirit
An useful comparative article on subclinical mastitis for farmers and field vets
A detailed article useful to farmers researchers and the field vets alike.