Abstract
Streptococcus suis is considered among the top bacterial pathogens leading to important economic losses to the swine industry, with the incidence of disease increases as the prophylactic use of antimicrobial is being vanished worldwide. S. suis is also a zoonotic agent afflicting people in close contact with infected pigs or pork meat. In man infections occurred in backyard farmers who were directly exposed to infection during the slaughtering process of pigs that had died of unknown causes or been killed for food because they were ill. S. suis infection may occur in humans, the most common clinical manifestations included purulent meningitis, septicemia, arthritis, and endocarditis. Control measures included prohibiting by the law of slaughtering, eating, selling, and transporting deceased or sick pigs. Public education campaigns were staged to increase awareness of how to prevent and control human S. suis infections. Surveillance systems should be established to alert farmers and the general public if an infection outbreak in pigs is recognized.
Keywords: Pigs, Humans, Meat, Slaughter, Meningitis

Introduction
Emerging zoonotic diseases have gained growing significance as a result of their detrimental effect on state, national, and international human and economic strategies in the distribution of public and animal health services. Streptococcus suis(S. suis) is a worldwide pig pathogen, but also a major developing zoonotic pathogen causing serious human systemic infection. Exposure to live pigs or the consumption of infected pork products may infect humans (Dutkiewicz et al., 2017). S.suis Trigger meningitis, with a high incidence of auditory complications secondary to labyrinthitis development, and often septic shock, with the primary source of infection being the pig (Lun et al., 2007). One the community-acquired bacterial meningitis is a dangerous illness with high mortality risk. The disease is described as a neurological emergency. Early diagnosis and targeted care are also of critical significance.
History
- suis was first recorded by veterinarians in 1954, after outbreaks of meningitis, septicemia, and purulent arthritis among piglets (Gustavsson et al., 2014). Fourteen years old, there was the first human S. suis cases have been diagnosed in Denmark and subsequent cases have been recorded in other northern European countries and Hong Kong (Zalas-Więcek et al., 2013). The number of humans. S. suis cases reported in the literature have risen dramatically over the last few years (Rajahram et al., 2017).
Microbiology
- suis is a gram-positive coccus that is mostly seen in pairs but may also be single or small chains. Determining S. suis biochemical studies, such as optochin, Voges-Proskauer, salicin, trehalose, and 6.5% sodium chloride, identifies S. suis to the species level. It is also possible to use commercial programs (e.g. API Strep; Biomerieux) (Chatzopoulou et al., 2015). A variety of possible virulence factors associated with S. suis Capsular polysaccharide, extracellular protein factor, the protein produced by muramidase, suilysin, multiple adhesins, hyaluronatelyase, and surface antigen were identified (Dutkiewicz et al., 2018).
Diversity of S. suis
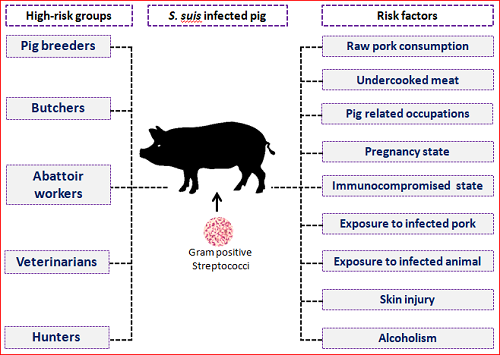
There are 35 known serotypes of this organism (Luque et al., 2010; Wang et al., 2013). Just a small number of the 35 known serotypes are responsible for pig infections, including serotypes 1-9 and 14, (Gottschalk et al., 2013). For both humans and pigs, Serotype 2 is recognized as the most pathogenic (Mancini et al., 2016). The two main serotypes of pathological importance to man are serotype 14 and serotype 2 (Gottschalk et al., 2013). Human cases are documented worldwide predominantly due to serotypes 2 (74.7%) and 14 (2.0%), all serotypes are similarly implicated in meningitis (50-70%) and septicemia (20-25%) cases (Goyette Desjardins et al., 2014). Serotypes 4,5,16,21,24 and 31 have been identified only in occasional cases (Callejo et al., 2014; Hatrongjitet al., 2015; Mancini et al., 2016). Many people affected with serotypes other than serotype 2 have pre-existing liver cirrhosis (Mancini et al., 2016) or other immunocompromising diseases (Callejo et al., 2014). This means that they could be less virulent than strains of serotype 2. Serotype 2 (27.9 %) is the worldwide prevalent serotype from diseased pigs, followed by serotypes 9 (19.4 %) and 3 (15.9 %) (Goyette-Desjardins et al., 2014).
Zoonotic outbreaks
The infection rates among the occupationally-exposed groups are poorly understood in most Western countries since S. suis infection is not a notifiable sickness (Feng et al., 2014). In Asian nations, the prevalence of occupational porcine streptococcosis is potentially considerably higher than in Europe (Segura et al., 2016). A total of 1642 human cases were registered worldwide until the end of December 2013 (Goyette-Desjardins et al., 2014), although this figure has since risen due to a significant number of recent case studies and the high risk of misdiagnosis. Most human cases have occurred in Asia (>90%), especially in Vietnam, Thailand, and China (Goyette-Desjardins et al., 2014). During the two outbreaks in China in 1998 and 2005, a total of 240 human cases were described (Meekhanon et al., 2017).
Threats to Asia
|
Country |
Clinical cases in Pigs from Jan 2002-Dec 2013 |
Reported cases in Human |
| North America | 3162 (67.1%) | 8 (0.5%) |
| South America | 125 (2.7%) | 9 (0.51%) |
| Asia | 659 (14.0%) | 1481 (90.2%) |
| Europe | 765 (16.2%) | 140 (8.5%) |
In Asia, where the vast majority of human cases have been registered, clinical case studies in pigs account for just 14.0% of the available evidence and are only provided by China and South Korea (Goyette-Desjardins et al., 2014). In India, epidemiological evidence and Scientific papers released are scanty. To deal efficiently with the infection of S.suis in pigs and humans, much data have to be generated.
Host and transmission
Hosts of natural reservoirs for S.suisPigs (Gottschalk et al., 2013)and wild boars are (Manzin et al.,2008). S.suis was also isolated from other species of wildlife, such as goats, deer, pigs, pigs, lambs, pigeons, catfish, and dogs (Muckle et al., 2014; Kerdsin et al., 2016). Besides, S.suis can be found in the slaughterhouse and wet market environments (Ma et al., 2008), especially in Southeast Asia, which is another human source of infection. Healthy pigs can bear multiple S.suis serotypes in their nasal cavities, tonsils, upper respiratory, reproductive, and food tracts. Horizontal transmission through the respiratory tract due to nose-to-nose touch is both a prevalent route and oral route (Schultsz et al., 2012), however vertical transmission from infected sow to piglets through the genital tract may often occur during farrowing(Mancini et al.,2016). Human S. suis infections are mainly due to recent contact with sick or asymptomatic pigs and the consumption of their contaminated meat derivatives. As a consequence, the virus occurs primarily in pig cattle employees and is known to be an occupational disease in many countries (Segura et al., 2015). Humans may become affected with S. suis by consuming raw or undercooked pork products (Onishi et al.,2012) or by skin lesions while in contact with infected pigs or polluted pork products (Gajdácset al., 2020). People in Asia are especially affected because high-risk dishes, such as raw blood pudding, TietCanh (Meekhanon et al., 2017), are popular and the pig industry is rising more and more, although people are not aware of the risks of infection(Okura et al., 2016). In western countries, only occasional cases of human infection arise due to contact with contaminated pigs or raw pork meat (Goyette Desjardins et al., 2014).
Clinical features
- suis diseases are considered to be multifactorial, adverse environmental factors that promote the growth of the disease(Gottschalk et al., 2013). Nasopharynx is a reservoir niche for S. suis and numerous other (potentially) pathogenic microorganisms and commensals (Opriessnig et al., 2011). In this niche, the commensals can behave as innocent by standermicrobes, which naturally colonize the respiratory mucosa, and may allow other optional pathogens to cause clinical disease (Gajdács et al., 2020). These facultative pathogenic species are known as pathobiota. If pathogens play a dominant role in microbiota changes in the population and additionally influence the host response, they are so-called keystone pathogens that can increase the virulence of pathobiota contributing to dysbiosis and inflammatory disease (Fittipaldi et al., 2011). For S. suis, synergistic actions with other bacterial agents, such as Pasteurella multocida or Mycoplasma hyopneumoniae, as well as respiratory viruses such as porcine reproductive and respiratory syndrome virus (PRRSV), porcine circo virus type 2, and swine influenza virus (SIV)(Blume et al., 2009)may increase the risk of invasive infections (Takeuchi et al., 2012). SIV and PRRVS are well-known keystone pathogens as they pave the way for S.suis infections leading to serious respiratory symptoms and severe pneumonia (del Rey et al., 2014)
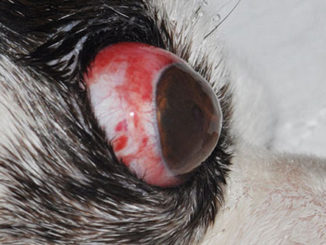
Susceptible pigs (especially weaning piglets) may suffer from meningitis, septicemia, pneumonia, endocarditis, or polyarthritis (Okura et al., 2016). Human S. suis most widely documented symptoms are septicemia, streptococcal toxic shock-like syndrome (STSLS) with multiple organ failure, endocarditis, cellulitis, rhabdomyolysis, pneumonia, arthritis, peritonitis, spondylodiscitis, uveitis, and endophthalmitis (Hughes et al., 2009; Aryasa et al., 2020). Arthritis affects various knees, including hips, knees, wrists, sacroiliacs, spine, and thumbs, and in most cases represents systemic septicemia caused by S. suis (Mancini et al.,2016; Fittipaldi et al., 2011). The most typical histopathological observations are found inside the choroid plexus (Prince-David et al., 2016; Votsch et al., 2018). In the central nervous system, lesions associated with meningitis and choroiditis, including leptomeningeal edema and dura mater, hyperaemic meningeal blood vessels, and an elevated volume of cerebrospinal fluid, can be found (Kerdsin et al., 2017). The most characteristic histopathological lesion of acute S. suis meningitis is diffuse neutrophil infiltrate (Luque et al., 2010; Wang et al., 2013).
Diagnosis
Human contamination with S. suis is currently under-reported and under-diagnosed in many countries due to a lack of understanding in the general population and physicians (Chidi et al., 2020). Several investigators have indicated that the unusual diagnosis of human S. suis infection is due to underdiagnosis or misdiagnosis, rather than a genuine loss of illness(Zhu et al., 2013; Lachance et al., 2013). S. suis infection diagnosis is based on distinctive macroscopic lesions, bacterial colonies, physiological, serological, and molecular characterization (Ho et al., 2011). Early diagnosis and successful antibiotic treatment are crucial points for raising the survival rate.
Treatment
During clinical situations, it is important to administer adequate therapy for patients with history and symptoms suggestive of S. suis infections, even if laboratory verification of the diagnosis is not yet available or in case of negative cultures (due to previous administration of antimicrobials or misidentification) (Hatrongjit et al., 2015). S. suis meningitis typically reacts very well to treatment with high doses of intravenous penicillin (Takeuchi et al., 2012). The organism is occasionally immune to penicillin. However, after 24 hours of treatment, fever, cough, and pain of the neck are typically moderate and mute (Fittipaldi et al., 2011). S.suisstrains isolated from diseased pigs are more susceptible to β-lactam antimicrobials, aminoglycosides, enrofloxacin, novobiocin, and spectinomycin, and more than 87% of S.suisisolates are immune to tetracyclines, sulphonamides, macrolides, and clindamycin(Okura et al., 2016). In another study, elevated levels of tolerance to tetracycline (48.0-92.0 %), trimethoprim sulphonamide (3.0-51.5 %) and erythromycin (29.1-75.0 %) were found in several European countries (Mancini et al.,2016). Early application of dexamethasone tends to reduce the occurrence of hearing loss and neurological sequelae of patients in these cases (Martin et al., 2001).
Prevention and control of S. suis in humans
Infection prevention and control measures are currently the “best-buy” method available to control disease transmission, at least until an effective vaccine becomes available. As there is no effective S.suis vaccine for humans.
- Appropriate and safe handling of animals to avoid bites and injuries.
- Wear gloves when handling sick animals, animal tissues, body fluids, and waste.
- Washing hands, arms, and other exposed parts of the body through after each contact with pigs or pork meat.
- Keep animal environments sterile and disinfect devices during use on livestock or in animal areas.
- Consult the doctor promptly in case of febrile infection following exposure to pigs or pork meat.
- Do not feed, drink, make-up, or use nicotine products when treating livestock or in animal shelters, in animal care areas, or the laboratory field.
- Evitation to induce skin lesions during meat processing operations should be taken.
- Covering open wounds with a waterproof dressing.
- Keeping the uncooked pork apart from other cooked food.
- Cooking pork to a temperature of 70o C or until the juice is clear and not pink.
- Laundry of soiled clothes apart from your clothing and ideally in an animal shelter.
- Prompt management or removal of contaminated tissues and animals.
- In some countries, travelers should be mindful that dietary habits may pose a risk to suis infection
- Diagnostic Specimen Handling: Wear gloves. Discard your gloves and wash your hands before handling clean objects (e.g., telephone). Wear dedicated protective gear such as lab coats or blankets and boots or foot covers while treating animals.
- Veterinary Medical Waste: Disposal of animal waste, pathology waste, animal carcasses, bedding, sharps, and biologics should be included. Follow your local and state ordinances regulations.
- Pregnant and immunocompromised personnel: pregnant and immunocompromised workers are at greater risk. Patients should prevent physical contact with pigs or pigs when skin lesions are especially present on their hands.
- From a public health viewpoint, food safety education programs may be a successful way to improve public awareness of this disease, especially in regions where there is a close correlation between conventional domestic pig slaughter and the consumption of raw meat and meat products.
- High-risk population monitoring is increasingly necessary to minimize the spread of suis to humans.
Conclusions
Rapid diagnosis and successful intervention against this disease are sluggish in many developed countries. Morbidity and mortality due to zoonosis are often documented due to shortcomings in veterinary facilities, skills, testing laboratories, and surveillance capability. The majority of human S. suis published cases originate in Southeast Asia, where the disease may be considered endemic. This result can be explained by the high density of pigs in the area, non-preventive slaughtering activities, and the intake of uncooked or lightly cooked pig products. Mapping the density of pigs and human S. suis cases say where S. suis is likely to be present but has not been recorded to date. A case-control study to identify risk factors for S. suis infection is lacking and is urgently required because S. suis meningitis is one of the most common causes of adult meningitis in many countries. Increased knowledge of both clinicians and microbiologists is required to fully understand the value of S. suis as a human pathogen. Since the vaccine for human use is still unavailable, it is important to pursue research into the development of an efficient and healthy vaccine.
References
- Aryasa, I.A., Widiasari, N.P.A., Susilawathi, N.M., Fatmawati, N.N.D., Adnyana, I.M.O., Sudewi, A.A.R. and Tarini, N.M.A., 2020. Streptococcus suis meningitis related to processing and consuming raw pork during Balinese tradition, Mebat. Medical Journal of Indonesia, 29(1), pp.88-92.
- Blume, V., Luque, I., Vela Alonso, A.I., Borge, C., Maldonado, A., DomínguezRodríquez, L., Tarradas, C. and FernándezGarayzábal, J.F., 2009. Genetic and virulence-phenotype characterization of serotypes 2 and 9 of Streptococcus suis swine isolates. International microbiology: the official journal of the Spanish Society for Microbiology, 12(3), pp.161-6.
- Callejo, R., Prieto, M., Salamone, F., Auger, J.P., Goyette-Desjardins, G. and Gottschalk, M., 2014. Atypical Streptococcus suis in man, Argentina, 2013. Emerging Infectious Diseases, 20(3), p.500.
- Callejo, R., Zheng, H., Du, P., Prieto, M., Xu, J., Zielinski, G., Auger, J.P. and Gottschalk, M., 2016.Streptococcussuis serotype 2 strains isolated in Argentina (South America) are different from those recovered in North America and present a higher risk for humans. JMM case reports, 3(5).
- Chatzopoulou, M., Voulgaridou, I., Papalas, D., Vasiliou, P. and Tsiakalou, M., 2015. Third case of Streptococcus suis infection in Greece. Case reports in infectious diseases, 2015.
- Chidi, A.G., Boakye, Y.A., Nyarko, O.O., Gyabaah, S., Konadu, S.O. and Opoku, G., 2020. Meningitis Caused by Streptococcus Suis: A Neglected Emerging Zoonotic Pathogen in Ghana. J Infect Dis Pre Med, 8, p.208.
- del Rey, V.S., Fernández-Garayzábal, J.F., Mentaberre, G., Briones, V., Lavín, S., Domínguez, L., Gottschalk, M. and Vela, A.I., 2014. Characterisation of Streptococcus suis isolates from wild boars (Susscrofa). The Veterinary Journal, 200(3), pp.464-467.
- Dutkiewicz, J., Sroka, J., Zając, V., Wasiński, B., Cisak, E., Sawczyn, A., Kloc, A. and Wójcik-Fatla, A., 2017. Streptococcus suis: a re-emerging pathogen associated with occupational exposure to pigs or pork products. Part I–Epidemiology.Annals of Agricultural and Environmental Medicine, 24(4), pp.683-695.
- Dutkiewicz, J., Zając, V., Sroka, J., Wasiński, B., Cisak, E., Sawczyn, A., Kloc, A. and Wójcik-Fatla, A., 2018. Streptococcus suis: a re-emerging pathogen associated with occupational exposure to pigs or pork products. Part II–Pathogenesis. Annals of Agricultural and Environmental Medicine, 25(1), pp.186-203.
- Feng, Y., Zhang, H., Wu, Z., Wang, S., Cao, M., Hu, D. and Wang, C., 2014. Streptococcus suis infection: an emerging/reemerging challenge of bacterial infectious diseases?.Virulence, 5(4), pp.477-497.
- Fittipaldi, N., Xu, J., Lacouture, S., Tharavichitkul, P., Osaki, M., Sekizaki, T., Takamatsu, D. and Gottschalk, M., 2011. Lineage and virulence of Streptococcus suis serotype 2 isolates from North America. Emerging infectious diseases, 17(12), p.2239.
- Gajdács, M., Németh, A., Knausz, M., Barrak, I., Stájer, A., Mestyán, G., Melegh, S., Nyul, A., Tóth, Á.,Ágoston, Z. and Urbán, E., 2020. Streptococcus suis: An Underestimated Emerging Pathogen in Hungary?.Microorganisms, 8(9), p.1292.
- Gottschalk, M., Lacouture, S., Bonifait, L., Roy, D., Fittipaldi, N. and Grenier, D., 2013. Characterization of Streptococcus suis isolates recovered between 2008 and 2011 from diseased pigs in Quebec, Canada. Veterinary microbiology, 162(2-4), pp.819-825.
- Gottschalk, M., Segura, M. and Xu, J., 2007. Streptococcus suis infections in humans: the Chinese experience and the situation in North America. Animal health research reviews, 8(1), p.29.
- Gottschalk, M., Xu, J., Calzas, C. and Segura, M., 2010. Streptococcus suis: a new emerging or an old neglected zoonotic pathogen?.Future microbiology, 5(3), pp.371-391.
- Goyette-Desjardins, G., Auger, J.P., Xu, J., Segura, M. and Gottschalk, M., 2014. Streptococcus suis, an important pig pathogen and emerging zoonotic agent—an update on the worldwide distribution based on serotyping and sequence typing. Emerging microbes & infections, 3(1), pp.1-20.
- Gustavsson, C. and Ramussen, M., 2014. Septic arthritis caused by Streptococcus suis serotype 5 in pig farmer. Emerging Infectious Diseases, 20(3), p.489.
- Hatrongjit, R., Kerdsin, A., Gottschalk, M., Takeuchi, D., Hamada, S., Oishi, K. and Akeda, Y., 2015. First human case report of sepsis due to infection with Streptococcus suis serotype 31 in Thailand. BMC infectious diseases, 15(1), p.392.
- Ho, D.T.N., Le, T.P.T., Wolbers, M., Cao, Q.T., Tran, V.T.N., Le, T.P.T., Nguyen, H.P., Tran, T.H.C., Dinh, X.S., To, S.D. and Hoang, T.T.H., 2011. Risk factors of Streptococcus suis infection in Vietnam. A case-control study.PloS one, 6(3), p.e17604.
- Hughes, J.M., Wilson, M.E., Wertheim, H.F., Nghia, H.D.T., Taylor, W. and Schultsz, C., 2009. Streptococcus suis: an emerging human pathogen. Clinical Infectious Diseases, 48(5), pp.617-625.
- Kerdsin, A., Gottschalk, M., Hatrongjit, R., Hamada, S., Akeda, Y. and Oishi, K., 2016. Fatal septic meningitis in child caused by Streptococcus suis serotype 24. Emerging infectious diseases, 22(8), p.1519.
- Kerdsin, A., Hatrongjit, R., Gottschalk, M., Takeuchi, D., Hamada, S., Akeda, Y. and Oishi, K., 2017. Emergence of Streptococcus suis serotype 9 infection in humans. Journal of microbiology, immunology, and infection= Wei mianyugan ran zazhi, 50(4), p.545.
- Lachance, C., Gottschalk, M., Gerber, P.P., Lemire, P., Xu, J. and Segura, M., 2013. Exacerbated type II interferon response drives hypervirulence and toxic shock by an emergent epidemic strain of Streptococcus suis. Infection and immunity, 81(6), pp.1928-1939.
- Lun, Z.R., Wang, Q.P., Chen, X.G., Li, A.X. and Zhu, X.Q., 2007. Streptococcus suis: an emerging zoonotic pathogen. The Lancet infectious diseases, 7(3), pp.201-209.
- Luque, I., Blume, V., Borge, C., Vela, A.I., Perea, J.A., Márquez, J.M., Fernández-Garayzábal, J.F. and Tarradas, C., 2010. Genetic analysis of Streptococcus suis isolates recovered from diseased and healthy carrier pigs at different stages of production on a pig farm. The Veterinary Journal, 186(3), pp.396-398.
- Ma, E., Chung, P.H., So, T., Wong, L., Choi, K.M., Cheung, D.T., Kam, K.M., Chuang, S.K., Tsang, T. and Collaborative Study Group on Streptococcus suis infection in Hong Kong, 2008. Streptococcus suis infection in Hong Kong: an emerging infectious disease?.Epidemiology & Infection, 136(12), pp.1691-1697.
- Mancini, F., Adamo, F., Creti, R., Monaco, M., Alfarone, G., Pantosti, A. and Ciervo, A., 2016. A fatal case of streptococcal toxic shock syndrome caused by Streptococcus suis carrying tet (40) and tet (O/W/32/O), Italy. Journal of Infection and Chemotherapy, 22(11), pp.774-776.
- Manzin, A., Palmieri, C., Serra, C., Saddi, B., Princivalli, M.S., Loi, G., Angioni, G., Tiddia, F., Varaldo, P.E. and Facinelli, B., 2008. Streptococcus suis meningitis without history of animal contact, Italy. Emerging infectious diseases, 14(12), p.1946.
- Meekhanon, N., Kaewmongkol, S., Phimpraphai, W., Okura, M., Osaki, M., Sekizaki, T. and Takamatsu, D., 2017. Potentially hazardous Streptococcus suis strains latent in asymptomatic pigs in a major swine production area of Thailand. Journal of Medical Microbiology, 66(5), pp.662-669.
- Muckle, A., Lopez, A., Gottschalk, M., López-Méndez, C., Giles, J., Lund, L. and Saab, M., 2014. Isolation of Streptococcus suis from 2 lambs with a history of lameness. The Canadian Veterinary Journal, 55(10), p.946.
- Okura, M., Osaki, M., Nomoto, R., Arai, S., Osawa, R., Sekizaki, T. and Takamatsu, D., 2016. Current taxonomical situation of Streptococcus suis. Pathogens, 5(3), p.45.
- Onishi, H., Sugawara, M., Okura, M., Osaki, M. and Takamatsu, D., 2012. Prevalence of Streptococcus suis genotypes in isolates from porcine endocarditis in East Japan. Journal of Veterinary Medical Science, pp.12-0301.
- Opriessnig, T., Giménez-Lirola, L.G. and Halbur, P.G., 2011.Polymicrobial respiratory disease in pigs. Animal Health Research Reviews, 12(2), p.133.
- Prince-David, M., Salou, M., Marois-Créhan, C., Assogba, K., Plainvert, C., Balogou, K.A., Poyart, C. and Tazi, A., 2016. Human meningitis due to Streptococcus suis in Lomé, Togo: a case report. BMC infectious diseases, 16(1), pp.1-4.
- Rajahram, G.S., Hameed, A.A., Menon, J., William, T., Tambyah, P.A. and Yeo, T.W., 2017. Case report: two human Streptococcus suis infections in Borneo, Sabah, Malaysia. BMC infectious diseases, 17(1), p.188.
- Schultsz, C., Jansen, E., Keijzers, W., Rothkamp, A., Duim, B., Wagenaar, J.A. and Van Der Ende, A., 2012. Differences in the population structure of invasive Streptococcus suis strains isolated from pigs and from humans in The Netherlands. PloS one, 7(5), p.e33854.
- Segura, M., 2015. Streptococcus suis vaccines: candidate antigens and progress. Expert review of vaccines, 14(12), pp.1587-1608.
- Segura, M., Calzas, C., Grenier, D. and Gottschalk, M., 2016. Initial steps of the pathogenesis of the infection caused by Streptococcus suis: fighting against nonspecific defenses. FEBS letters, 590(21), pp.3772-3799.
- Takeuchi, D., Kerdsin, A., Pienpringam, A., Loetthong, P., Samerchea, S., Luangsuk, P., Khamisara, K., Wongwan, N., Areeratana, P., Chiranairadul, P. and Lertchayanti, S., 2012. Population-based study of Streptococcus suis infection in humans in Phayao Province in northern Thailand. PloS one, 7(2), p.e31265.
- Vötsch, D., Willenborg, M., Weldearegay, Y.B. and Valentin-Weigand, P., 2018. Streptococcus suis–the “Two Faces” of a pathobiont in the porcine respiratory tract. Frontiers in microbiology, 9, p.480.
- Wang, K., Zhang, W., Li, X., Lu, C., Chen, J., Fan, W. and Huang, B., 2013. Characterization of Streptococcus suis isolates from slaughter swine. Current microbiology, 66(4), pp.344-349.
- Zalas-Więcek, P., Michalska, A., Grąbczewska, E., Olczak, A., Pawłowska, M. and Gospodarek, E., 2013. Human meningitis caused by Streptococcus suis. Journal of medical microbiology, 62(3), pp.483-485.
- Zhu, W., Wu, C., Sun, X., Zhang, A., Zhu, J., Hua, Y., Chen, H. and Jin, M., 2013. Characterization of Streptococcus suis serotype 2 isolates from China. Veterinary microbiology, 166(3-4), pp.527-534.










Be the first to comment