Abstract
Actinobacillus pleuropnemonia is a common primary bacterial pneumonia in swine populations in worldwide. Porcine Actinobacillus pleuropneumonia, caused by Actinobacillus (Haemophilus) pleuropneumoniae, is a highly contagious, fibrinous, hemorrhagic, and necrotizing pneumonia with high mortality or localized lung lesions in chronically infected pigs. Transmission of A. pleuropneumoniae among swine is commonly ascribed to exposure to contaminated respiratory secretions, and/or aerosols. It has a significant impact on animal welfare and economic production, due to high morbidity and mortality rates, the reduction of average daily weight gain and feed conversion rates, loss due to slaughter and intervention costs. Prelimanary diagnosis is based on clinical sign and gross pneumonic lesions. Isolating and identifying the causative bacteria confirms the diagnosis. Antibiotic therapy, vaccination, and test-and-saughter programs, either alone or in combination, have been used to try to control pleuropneumonia with varying degrees of success.
Introduction
Actinobacillus Pleuropnemonia (App) is a primary bacterial pathogen causing swine respiratory disease and considered as one of the most common pathogenic bacteria in the pig industry (Marsteller et al., 1999; Vigre et al., 2002) Pleuropneumonia is a severe and contagious respiratory disease, primarily of young pigs (≤6 mo old), although in an initial outbreak, adults also may be affected. It has a significant impact on animal welfare and economic production, due to high morbidity and mortality rates, the reduction of average daily weight gain and feed conversion rates, loss due to slaughter and intervention costs. Actinobacillus pleuropneumoniae is a widely studied bacterium; however its pathogenesis is not yet fully understood. It occurs worldwide and appears to be increasing in incidence. Swine pleuropneumonia caused by App and is a major problem for swine production. The disease is characterized by pneumonia, pleurisy, growth retardation and mortality in the affected population (Plasencia-Muñoz et al., 2020).
Etiology
Actinobacillus pleuropneumoniae (App) is a respiratory pathogen member of the family Pasteurellaceae and etiological agent of swine pleuropneumonia. App is gram negative, non-motile, non-spore forming, small coccoid or rod shaped bacteria (Vigre et al., 2002). To date, 15 serotypes have been identified. Historically, serotypes 1, 5, and 7 have been prevalent in the USA. Depending on its nicotinamide adenine dinucleotide (NAD) requirements, it is classified into two biotypes, biotype 1 groups NAD-dependent strains, biotype 2 groups NAD-independent strains. The biotypes and serotypes of A. pleuropneumoniae have genetic, structural, and virulence differences. Serotyping is mainly based on capsular antigens. The presence and prevalence of serotypes varies between countries (Plasencia-Muñoz et al., 2020).
Transmission
Actinobacillus pleuropneumoniae can be transmitted between herds through various vectors, including direct contact with infected animals, contaminated aerosols and fomites. Transmission is mainly by nose-to-nose contact, and many recovered pigs are carriers. Direct transmission from an infected to a susceptible pig appears to be the most frequent means for spreading the disease since these bacteria are highly susceptible to drying and common disinfectants. Hence, one strategy for controlling these losses is to exclude the disease by not admitting infected pigs into the herd. Survivors of acute infection often develop chronic lung lesions (sequestra, abscesses, and pleuritic adhesions) and become carriers. Chronically infected animals have reduced feed conversion, higher medication costs, lower weaning rates, and reduced market value (Prodanov-Radulović et al., 2020).
Pathology
Actinobacillus pleuropneumoniae is described as a strict extracellular pathogen with tropism for lower respiratory tract. APP quickly colonizes the host animal, first by attaching to the epithelial cells of the tonsils and then moving to the lower respiratory tract where it creates the majority of damage. As the bacteria multiplies, it releases particles of an outer membrane that contain lipopolysaccharides (LPS) and cytotoxins. Neutrophils arriving as the beginning of an inflammatory response are attracted by the LPS and are then destroyed by the cytotoxins. As the neutrophils are destroyed, lysozymes are released, damaging nearby tissue. The tissue damage can progress rapidly, and some animals that are infected with APP will die within 4-12 hours of exposure. The LPS and cytotoxins not only help the bacteria damage the host cells, they also prevent the bacteria from being destroyed by impairing phagocytosis and complement activity (Bosse et al., 2002)
Clinical findings
Onset is sudden, and in herds that have not been infected previously, spread is rapid. Some pigs may be found dead without having shown clinical signs. Respiratory distress is severe; there are “thumps,” and sometimes open-mouth breathing with a blood-stained, frothy nasal and oral discharge. Fever up to 107°F (41.5°C), anorexia, and reluctance to move are typical signs. Although primarily a disease of growing pigs, A. pleuropneumoniae infection may be fatal in adults or cause sows to abort. The course of the disease varies from peracute to chronic. Morbidity may reach 50%, and in untreated cases, mortality is high. Survivors generally show reduced growth rates and persistent cough.
Once established in a herd, the disease may be evident only as a cause of reduced growth rate and pleurisy at the abattoir, although acute disease exacerbations may occur. However, severe lesions may not always be accompanied by equally severe clinical signs. Deaths in transit and carcass condemnation may result. Concurrent infection with mycoplasma, pasteurellae, porcine reproductive and respiratory syndrome, or swine influenza virus is common.
Lesions
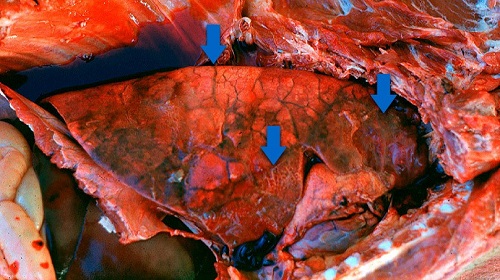
Necropsies of acutely infected swine are the best way to diagnose an outbreak of Actinobacillus pleuropneumoniae. Pleuropneumonia that is fibrinohemorrhagic and necrotizing is usually seen bilaterally in the lungs. It is often focal and found in the caudal lung lobes, but it can be diffuse and may be seen in any or all parts of the lung lobes. The lungs may be darkened and consolidated, and pleural adhesions and abscesses are often found in chronic cases. Fibrin is commonly found throughout the surface of the lungs, and peracutely affected animals may have foamy, bloody exudate in the trachea and bronchi. When viewing the animal’s clinical signs and gross lesions, additional differential diagnoses tend to include diseases that affect the respiratory system and show signs of septicemia such as Actinobacillus suis, Haemophilus parasuis, and Salmonella cholerasuis. Microscopic examination of APP lesions usually shows severe fibrinohemorrhagic pneumonia that is often suppurative and necrotizing (Gottschalk et al., 2006). Vasculitis, pleuritis, and bronchiolitis are often additional components of the disease. Neutrophils are seen more often in an early course of APP, while, later in the disease, macrophages are increased and fibrosis is more severe. Definitive diagnosis is usually confirmed with the aid of histopathology and bacterial cultures.

Diagnosis
Prelimanary diagnosis is based on clinical sign and gross pneumonic lesions. Isolating and identifying the causative bacteria confirms the diagnosis. An explosive disease onset is suggestive and, when combined with clinical signs and gross lesions, often justifies a tentative diagnosis. Concurrent infections with pasteurellae may complicate diagnosis. In herds that have been exposed and have developed at least a degree of immunity, the pattern may be less distinctive. Many serologic tests, including complement fixation and ELISA, have been used to confirm a herd diagnosis or detect carriers, but results are not always straightforward. A definitive diagnosis depends on isolation and identification of A. pleuropneumoniae, a gram-negative coccobacillus that requires V factor (NAD) supplementation for growth. A Staphylococcus aureus nurse colony can provide the necessary factor. PCR testing is also available and provides better sensitivity than direct culture (MacInnes et al., 1988).
Treatment
Rapidity of onset and persistence in infected herds make treatment difficult. The first treatment should be parenteral, followed by medication given in water or feed, which also may protect contact pigs. Long-acting oxytetracyline, given by injection, may prevent pleuropneumonia in pigs exposed to infection and can reduce mortality if used early during clinical disease. Tiamulin, a drug commonly used against porcine enzootic pneumonia, has also been reported to significantly improve the performance of pigs with pleuropneumonia (Huang et al., 1999). Antibiotic therapy is, however, of no benefit to pigs with chronic pleuropneumonia.
Prevention and Control
Vaccination: There are different types of commercial vaccines available. These vaccines can be classified as:
- Bacterins (washed and killed whole bacteria): Protection given by this type of vaccine is serotype-specific: you need to know which serotype is involved in the farm. It is unusual (not impossible when different origins are mixed) to have more than one serotype causing disease in a single farm: a good diagnosis is needed. Antibodies raised against this vaccine are directed to “bacterial body antigens”: capsule, surface proteins, bacterial cell wall, etc. When these antibodies are present in the animal lung, they “attach” to bacteria and alveolar macrophages and other cells will ingest and destroy App. In the absence of these antibodies, phagocytic cells cannot “capture” App bacteria, which reproduce and produce, among other products, the toxins and cause lesions.
- Purified toxoid-based vaccines (sometimes enriched with surface proteins): ApxI, ApxII and ApxIII toxoids are present. Different strains of App produce one or two of these toxins. Protection given by this type of vaccine is usually against all serotypes, since all App strains produce one or two of the toxins included. Antibodies raised against this vaccine “neutralize” the toxins: just prevent them to cause lesions. Antibodies do not react with App bacteria: toxins are secreted. So, App can still reproduce in the lungs, although toxic effect of toxins is neutralized.
- “Mixed”: Bacterin (for specific serotypes) + purified toxoid-based vaccine: it is a combination of “1” and “2”. Antibodies produced are anti-toxins and anti-bacterial components (only for serotypes included in the vaccine) (Kansas State University, updated: 12/20/19)
Conclusion
Due to this great economic impact, prevention and control programs for pleuropneumonia should receive high priority. Because survivors frequently remain carriers, control is difficult, although good results are being claimed for some vaccines. Segregated early weaning, “all-in/all-out” management, reduced stocking rates when possible, and improved ventilation are recommended. In herds free of the disease, replacements should be purchased from herds free of A pleuropneumoniae; if the disease proves difficult to control, herd depopulation and repopulation should be considered. Serologic testing effectively detects previously infected herds but may not identify carrier animals. Antibiotic therapy, vaccination, and test-and-slaughter programs, either alone or in combination, have been used to try to control pleuropneumonia with varying degrees of success.
References
- Prodanov-Radulović, J., Vučićević, I., Polgek, V. and Aleksić-Kovačević, S., 2020. Current swine respiratory diseases morphology in intensive swine production in Serbia. Acta Veterinaria-Beograd, 70(1), pp.1-36.
- MacInnes, J.I. and Rosendal, S., 1988. Prevention and control of Actinobacillus (Haemophilus) pleuropneumoniae infection in swine: a review. The Canadian Veterinary Journal, 29(7), p.572.
- Willson, P.J., Falk, G. and Klashinsky, S., 1987. Detection of Actinobacillus pleuropneumoniae infection in pigs. The Canadian Veterinary Journal, 28(3), p.111.
- Huang, H., Potter, A.A., Campos, M., Leighton, F.A., Willson, P.J. and Yates, W.D., 1998. Pathogenesis of porcine Actinobacillus pleuropneumonia: Part I. Effects of surface components of Actinobacillus pleuropneumoniae in vitro and in vivo. Canadian journal of veterinary research, 62(2), p.93.
- Huang, H., Potter, A.A., Campos, M., Leighton, F.A., Willson, P.J., Haines, D.M. and Yates, W.D., 1999. Pathogenesis of porcine Actinobacillus pleuropneumonia, part II: roles of proinflammatory cytokines. Canadian journal of veterinary research, 63(1), p.69.
- Plasencia-Muñoz, B., Avelar-González, F.J., De la Garza, M., Jacques, M., Moreno-Flores, A. and Guerrero-Barrera, A.L., 2020. Actinobacillus pleuropneumoniae interaction with swine endothelial cells. Frontiers in Veterinary Science, 7.
- Marsteller, T.A. and Fenwick, B., 1999. Actinobacillus pleuropneumoniae disease and serology. Journal of Swine Health and Production, 7(4), pp.161-165.
- Vigre, H., Angen, Ø., Barfod, K., Lavritsen, D.T. and Sørensen, V., 2002. Transmission of Actinobacillus pleuropneumoniae in pigs under field-like conditions: emphasis on tonsillar colonisation and passively acquired colostral antibodies. Veterinary microbiology, 89(2-3), pp.151-159.
- Bossé, J.T., Janson, H., Sheehan, B.J., Beddek, A.J., Rycroft, A.N., Kroll, J.S. and Langford, P.R., 2002. Actinobacillus pleuropneumoniae: pathobiology and pathogenesis of infection. Microbes and infection, 4(2), pp.225-235.
| The content of the articles are accurate and true to the best of the author’s knowledge. It is not meant to substitute for diagnosis, prognosis, treatment, prescription, or formal and individualized advice from a veterinary medical professional. Animals exhibiting signs and symptoms of distress should be seen by a veterinarian immediately. |











Be the first to comment