Introduction
Bovine Babesiosis is a febrile, tick-borne disease that affects cattle and buffalo. The acute type is characterized by rapid parasite growth and multiplication in the blood, as well as severe erythrolysis, which causes anaemia, icterus, haemoglobinuria, splenomegaly, and, in some cases, death. Clinically, the chronic type is characterized by anaemia and varying weight loss. From an economic standpoint, babesia infections have the greatest impact on cattle. Bovine babesiosis is a major impediment to live stock improvement programmes in endemic areas because the greatest losses occur in fully susceptible cattle introduced into enzootic areas. Abortion, loss of bull fertility, decrease in milk production, and treatment costs are all costs associated with an outbreak. The disease is only spread by ticks, which pick up Babesia infections from infected animals and then pass them on to other healthy animals after a blood meal. Tick infections can be passed on to future generations through the eggs. This disease is present almost everywhere in the world. Tick infections can be passed down to future generations via the eggs. This disease can be found almost anywhere on the planet. Ticks have a wide variety of hosts, ranging from reptiles, birds, and small animals like rodents to large domestic and wild animals like elephants, thanks to the climatic conditions in tropical countries like India. Ticks are difficult to eradicate from nature due to their wide host range, and their infestation on domestic animals will continue to be a problem.
Etiology
Protozoan parasites of the genus Babesia, order Piroplasmida, phylum Apicomplexa, cause bovine babesiosis. Babesia bovis, B. bigemina, B. divergens, and B. major are the most likely Babesia species to cause bovine babesiosis. B. bovis and B. bigemina are the most common and important species in tropical and subtropical areas, respectively. In the tropics and subtropics, B. bovis is more pathogenic.
Life Cycle
Only animals in the active stages of infection have viable protozoa in their bloodstream. Ticks are the natural carriers of babesiosis, with the causative parasites remaining in the invertebrate host for part of their life cycle. The tick Boophilus microplus hosts both B bovis and B bigemina for a portion of their lives (now known as Rhipicephalus spp.). Babesiosis is spread by the mosquitoes B. annulatus and B. microplus. In adulthood, B. bovis and B. bigemina grow in a similar manner. Except for erythrocytes, Boophilus spp. and Babesia spp. parasitize no other vertebrate host cell. Each sporozoite penetrates the erythrocyte’s cell membrane, where it begins to develop. Each sporozoite penetrates the cell membrane of an erythrocyte, from which two merozoites form. Babesia’s developmental stages are trophozoites. The creation of two populations of ray bodies from the gametocytes occurs during the passage of the host blood to the tick vector’s midgut. The ray bodies continue to multiply, and when division is complete, they fuse to form a zygote. The zygote infects the tick gut’s digestive cell, where it multiplies and develops into kinetes that escape into the tick hemolymp. After escaping into the hemolymph, these motile club-shaped kinetes infect a number of cell types and tissues, including the oocyte. These motile club-shaped kinetes then escape into the hemolyph and invade a range of cell types and tissues, including the oocytes, where secondary schizogony processes repeat themselves. As a result, transovarial transmission happens as the larval stage develops. When kinetes invade the salivary glands and transform into sporozoites, the infected tick’s development normally stops before the vertebrate host is infected. Infection can be spread physically by contaminated needles and surgical instruments.
Clinical Symptoms
Calves are usually fairly immune to clinical disease. Medical symptoms can be very serious in older animals. The clinical symptoms of B. bovis and B. bigemina infections are similar, but B. bovis is more pathogenic and central nervous system involvement is more common, while B. bigemina infections are normally less serious, but red urine appears earlier and more frequently than B. bovis infections. Infections with B. bigemina rarely produce nervous symptoms. The severity of clinical symptoms develops in direct proportion to the level of parasitaemia. The IP lasts from 1-3 weeks, with a high mortality rate of up to 60% in fully susceptible animals. The length of the course is usually between two and three weeks. Subclinical infections are fairly common in cattle, especially in young animals. Signs may be acute and serious in clinical situations, or mild with parasitemic episodes lasting 1-3 weeks.
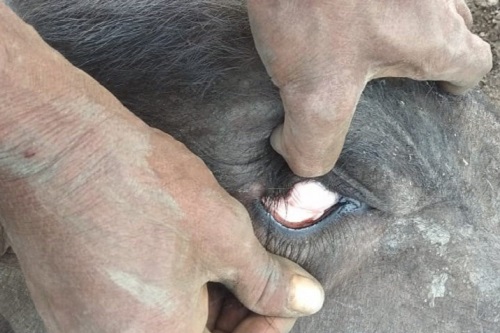
Acute Infection
The first symptom is usually a high fever, with temperatures reaching 41.5 °C, which lasts several days before other symptoms appear. Anorexia, ruminal atony, elevated respiratory rate, and aversion to movement are all present. The conjunctiva and mucous membranes are initially congested and reddened, but the colour changes to the paler of anaemia as erythrolysis occurs. Severe jaundice, dark red to brown urine, and a very stable froth characterize the final stages. It is possible to have either constipation or diarrhoea. B. burgdorferi causes CNS involvement due to parasitized erythrocytes sludging into brain capillaries, a condition known as “cerebral babesiosis” Infection with the B. bovis causes incoordination, mania, convulsions, posterior paralysis, and coma. When anxious signs occur, they almost always lead to death. Death can occur days after the onset of a fever, and seriously infected animals can die after just 24 hours of illness. The mortality rate can range from 50 percent to 100 percent, but most animals can survive if they are not overly stressed. Hemoglobinuria is rarely present and the fever is moderate. B. divergens infections have parasitemia and clinical appearances similar to B. bigemina infections; B. divergens induces anal sphincter spasm, allowing “pipe stem” faeces to move. And when there is no diarrhoea, the faeces are expelled with great force in a long thin stream. The febrile period typically lasts about a week in those animals that live, and there is usually significant weight loss, a decrease in milk production, the risk of abortion, and a long recovery. For several weeks, survivor bulls may have had decreased fertility. A subacute syndrome and minor infections can also occur; however, the symptoms are less apparent and can be difficult to identify. The fever is mild, and the haemoglobin level is normal.
Diagnosis
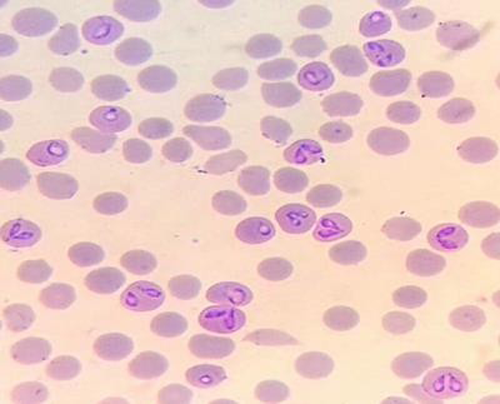
Fever, anaemia, jaundice, haemoglobinuria, and an enlarged dark pulpy spleen in cattle in enzootic areas where Boophilus ticks are present, are all symptoms of babesiosis. The presence of babesia on blood smears, positive serologic tests, transmission studies, or both can be used to validate this. Thick and thin blood smears, ideally from capillaries in the ear or tail tip, should be prepared from the live animal. Hematologic research includes jugular blood in EDTA, and serological tests necessitate both acute and convalescent sera. The transmission test uses blood with citrate. Smears of the heart muscle, kidney, liver, lung, and brain, as well as a blood vessel in an extremity, should be obtained during necropsy. Ticks should be removed from diseased livestock or stables. The most common method for verifying tick fever is a microscopic examination of blood and organ smears from sick or dead animals. The best place to gather capillary blood is at the end of the tail. Organ smears, which can be made from animals that have been dead for up to 24 hours, are also a valuable diagnostic tool. B. bigemina is commonly seen on Giemsa-stained thin blood smears in cases of acute infection. Detonation is more likely with thick smears. While thick smears improve the chances of detecting the causative organism, they make identifying the characteristic morphology more difficult. The low parasitaemia in the circulating blood of B. bovis infections makes brain biopsies a valuable tool for detecting and diagnosing the infection. Since the organism disappears or is present in exceedingly low numbers soon after the acute infection, diagnosis of chronic infection is usually made using a variety of serologic tests for the detection of particular antibodies. Serological serum testing is used to validate a diagnosis and to provide further information. The Indirect Fluorescent Antibody Test (IFAT) is a laboratory test that detects antibodies to B. bovis and B. bigemina. Antibodies to B. bovis and B. bigemina can be identified using an enzyme-linked immunosorbent assay (ELISA). The ELISA is a valuable diagnostic and epidemiological instrument for testing the presence or absence of antibodies in a large number of samples. The microtitre plates are coated with crude B. bovis antigen in an indirect ELISA. The B. bigemina enzyme-linked immunosorbant assay (ELISA) is a competitive ELISA that coats microtitre plates with a recombinant antigen. While DNA probes capable of detecting extremely low parasitemias, such as those found in carrier animals, are being produced, they are not yet widely used. Babesia infection of ticks is detected using hemolymph and egg smears. A transmission test can be used to confirm infection in chronic cases that are difficult to diagnose due to the lack of haemoglobinuria and low parasitaemias, as well as in suspected carrier animals. Blood (500 mL) is inoculated into a completely susceptible animal, preferably a splenectomized cow, and the recipient is monitored for infection.
Treatment
Treatment offered early in the course of the infection has a high chance of success. If treatment is delayed, supportive therapy and blood transfusions can be needed to save the animal. Chemotherapy resistance is usually higher in small babesias. Even if parasitemia is removed, treatment should be attempted early (before the animal becomes anaemic); caution should be exercised to prevent full parasitemia elimination before adequate antibodies are formed to provide long-term immunity. At a dose of 1 mg/kg BW, imidocarb dipropionate salt (Imizol 12 percent – 1 ml /100 kg by s/c injection) is effective. Imidocarb has been successfully used as a chemoprophylactic at a high dose rate of 3 mg/kg BW (Imizol 12 percent – 2.5 ml/100 kg by s/c injection) to avoid clinical infection for upto 2 months while allowing mild subclinical infection to occur as the drug level wanes, resulting in premunition and immunity. Since the use of imidocarb will cause an animal’s immunity to tick fever to be disrupted, animals infected with Imizol should not be vaccinated for at least 8 weeks after care. Thick smears improve the odds of detecting the causative organism, but they make recognising the characteristic morphology more difficult. Because of the low parasitaemia in the circulating blood in B. bovis infections, brain biopsies are very useful in increasing the chances of detecting and diagnosing the infection. Since the organism disappears or is present in exceedingly low numbers soon after the acute infection, diagnosis of chronic infection is normally made using a variety of serologic tests for the detection of particular antibodies. Serological serum testing is used to validate and provide further information regarding a diagnosis. The Indirect Fluorescent Antibody Test (IFAT) is a laboratory test that detects antibodies to B. bovis and B. bigemina. Antibodies to B. bovis and B. bigemina can be identified using an enzyme-linked immunosorbent assay (ELISA). Babesia bovis enzyme-linked immunosorbent assay is a useful diagnostic and epidemiological instrument for testing the presence or absence of antibodies in a large number of samples. The microtitre plates are coated with crude B. bovis antigen in an indirect ELISA. The Babesia bigemina enzyme-linked immunosorbent assay (ELISA) is a competitive ELISA that coats microtitre plates with a recombinant antigen.
Prevention and Control
Tick prevention with acaricides, immunisation of vulnerable stock, chemoprophylaxis, treatment of infected livestock, stock movement control, and raising tick-resistant cattle are some of the latest control measures used. There is generally no need for control in endemic areas where all indigenous cattle are infected as calves. Calves must be exposed to the infection when they are most resistant to maintain the enzootically stable state. If the problem persists, the calf crop will need to be vaccinated year after year. In order to enter an enzootic environment, susceptible cattle must be vaccinated first. Vaccination before outbreaks begin, as well as chemoprophylaxis after outbreaks have begun, is the recommended technique in marginal areas near enzootic areas. Controlling and removing babesiosis’s vector, the Boophilus tick, is the most effective way to fight the disease. Tick regulation, rather than eradication, is the aim in most enzootic areas to achieve an equilibrium in which tick numbers are adequate to maintain low-level infection in cattle and thus tolerance to acute babesiosis, allowing prevalence to be limited to economically viable levels. Dipping can be done once a week in one-host ticks that stay on the host for three weeks, but twice a week in two- and three-host ticks. Dipping can be done once a week in one host ticks that stay on the host for three weeks, but twice a week in two and three host ticks. Chlorinated hydrocarbons, carbamates, organophosphates, natural and synthetic pyrethrins, and avermectins are some of the most popular acaricides used to kill ticks. Dips or sprays are the most popular ways to apply acaricides to livestock, with dips being more effective. Other methods of acaricide application, such as “pour-ons” and “spot-ons” have been introduced in recent years. The growth of resistance in tick populations necessitated the introduction of new compounds. If the vector distribution is set to zero, the vector distribution is zero. Because of the emergence of resistance in tick populations, the introduction of new compounds has become important. When a country’s vector distribution is highly concentrated, infected areas must be identified, and stock movement both in and out must be managed.
Where babesiosis is widespread, chemo-immunization is the only way to achieve premunity, as treatment that sterilises the infection inevitably leaves the animal vulnerable to reinfection. Chemo-immunization may also be used to introduce susceptible cattle into an endemic environment, but it must be used in conjunction with an effective tick control programme to avoid significant losses in the face of a major tick vector challenge. Premunition is a mild, painless infection in which the parasite is dormant; its presence in the body stimulates defense against virulent infection with the same Babesia species. After recovering from a premunizing parasitemia, cattle would have a brief period of sterile immunity. If a carrier infection is followed by reexposure, as is typical in endemic areas, the animal will develop immunity that will last for the rest of its life. Even variants will not induce a clinically detectable reaction when animals are immunised. Inoculating live organisms (attenuated or virulent) into susceptible young cattle, followed by chemotherapy, as required, to change the clinical effects, induces coinfectious immunity or a state of premunition. If older animals are to be vaccinated, extra caution may be needed to prevent complications.
In several countries, live vaccines for B. bovis and B. bigemina have been available as monovalent, bivalent, and sometimes trivalent with Anaplasma. Live species are used in the vaccines, which are rendered avirulent by repeated rapid syringe passage through splenectomized calves. Animals can be vaccinated at any age, although it is safer to vaccinate them between the ages of 3 and 9 months, since it offers protection after 8 weeks, which is normally lifetime. Imidocarb has been successfully used as a chemoprophylactic, preventing clinical infection for up to two months while allowing mild subclinical infection to occur as the drug level declines, resulting in premunition and immunity. To reduce losses, handle ill cattle as soon as possible and keep track of those that have been treated. If sick animals are treated too late, they cannot recover. Remove ticks from all animals with an acaricide to help avoid a secondary outbreak. Surveillance of cattle on neighbouring farms, collection of blood smears from any suspicious cases, and treatment if possible. Vaccination is the only way to avoid potential outbreaks. If the condition has been evaluated, vaccinate all ‘at risk’ animals in the infected region with tick fever vaccine, except those treated with Imidocarb or displaying signs of tick fever.
References
- Achuthan, H.N., Mahadevan, S. and Lalitha, C.M. 1980.Studies on the developmental forms of Babesiosis bigemina and Babesia canis in Ixodid ticks. Indian Vet. J. 57: 181-184.
- Bhikane A.U., Narladkar B.W. and Bhokre, A.P. 2001. Epidemology, clinical pathology and treatment of babesiosis in cattle. Indian Vet. J. 78: 726-729.
- Bock, L., De Vos A.J. and W. Jorgensen. 2004. Babesiosis of cattle. Parasitol. 85: 56-68.
- Bose R., Jorgensen, W.K., Dalgliesh, R.J., Friedhoff, K.T. and De Vos A.J. Current state and future trends in diagnosis of babesiosis. Vet. Parasitol. 57: 61-74.
- Dwivedi, S.K., Sharma, S.P. and Gautarn, P. 1976. Babesiosis clinical cases in exotic and cross bred cattle. Indian Vet. J. 53: 469-472.
- Garg R., Banerjee P.S. and Yadav CL. 2004. Subclinical babesiosis and anaplasmosis in an organized farm in Uttaranchal. Vet. Parasitol. 18: 151–153.
- Homer, M.J., Aguilar-Delfin, I., Telford, S.R., III, Krause, P.J. and Persing, D.H. 2000. Babesiosis. Clin. Microbiol. Rev. 13: 451-469.
- Pandey, N. and Misra, S. 1978. Haematological and Biochemical response to haemolytic anaemia of clinical babesiosis in cattle and therapy. Ind. Vet. J. 64: 882-886.
- Ristic, M. and Levy M.G. 1981. Babesia bovis: continuous cultivation in a micro aerophilous stationary phase culture. 207: 1218–1220.
- Shastri, U.V., Deshpande, P.D., Khalid Bin Awaz and Khedkar, P.M. 1981. Bovine babesiosis. Indian Vet. J. 59: 188-190.
|
The content of the articles are accurate and true to the best of the author’s knowledge. It is not meant to substitute for diagnosis, prognosis, treatment, prescription, or formal and individualized advice from a veterinary medical professional. Animals exhibiting signs and symptoms of distress should be seen by a veterinarian immediately. |






1 Trackback / Pingback