INTRODUCTION
Livestock is one of the fastest growing agricultural subsectors in developing Countries including India. The demand for dairy products like milk and milk products is rapidly increasing in India. Animal health plays an important role in harnessing the expected production potential of dairy animals.
A premix is a mixture of vitamins, trace minerals, medicaments, feed supplements and diluents. It is a value added solution for feeds with sustainable safety and quality. The premix industry is charged with the responsibility of manufacturing a high quality premix consistently, efficiently and economically. Premixes contain vitamins, microminerals and other allowed feed supplements, which the animals need for a healthy life and good breeding results. Premixes come in the form of strong concentrates. Only a small percentage (0.5%, 1% or 2 %) is added to feed mixture. Registered and approved plants engage in feed production and management.
PREMIXES CONTAINS
VITAMINS: Vitamins are chemical substances, needed by the organism in small quantities; however, they are essential for the correct course of numerous chemical reactions, which enable a normally functioning of organism.
Example: A, D3, E, K3, B1, B2, B6, B12, C, nicotinic acid, calcium pantothenate, folic acid, biotin and choline chloride.
MICROMINERALS: Microminerals are elements which the organism needs only in small quantities. Iodine (I), manganese (Mn), zinc (Zn), iron (Fe), copper (Cu), cobalt (Co), selenium (Se), molybdenum (Mo) and sulfur (S),
AMINO ACIDS: Amino acids are the bricks of proteins, the organism needs them in order to grow and develop. Different proteins contain different amino acids in different sequences and numbers. Some amino acids are synthesized by microorganisms in animal’s gastrointestinal tract or they are synthesized from other amino acids. But some amino acids have to be acquired through food – essential amino acids. They are lysine, methionine, threonine, tryptophan, arginine, histidine, leucine, isoleucine, phenylalanine and cysteine.
ENZYMES: Enzymes are essential for decomposition of feed and they make possible the absorption of nutritive substances from feed. Animals can produce some enzymes themselves, but there are some that they cannot produce. The nutrients in feed cannot be properly absorbed and feeding becomes more expensive in this way.
PHYTASE: decomposes phytate complexes and improves the absorption of phosphorus.
XYLANASE: decomposes arabinoxylane (non-starch polysaccharides) in cereals (wheat, barley, rye, triticale, oats) which increases the viscosity of substances in the gastrointestinal tract and consequently reduces the absorption of nutrients. Xylanase breaks bonds within the long chains of arabinoxylanes, the chains become shorter and the formation of gels is reduced. At the same time nutrients tied to the complex are released and they become available for digestion.
Gluconase decomposes ß-glucan, which forms an integral part of cellular walls in animal feed. They are used in order to improve the digestibility of protein rich vegetable feed (soya, rape, beans and sunflower seeds) for poultry and pigs. They operate on the same principle as xylanase.
COCCIDIOSTATS: Coccidiostats are antibiotics and chemotherapeutics, which prevent the occurrence of coccidiosis. A modern breeding of broilers and turkeys would be impossible without them. Coccidiosis is caused by one cell parasites which invade the intestinal wall of animals. The development and reproduction of parasites in the intestinal wall causes damage. If we do not stop it, mild diarrhea or inhibited growth can appear. Ultimately, animals can also die from it, which are given to animals to prevent the development of coccidiosis. They are essential for the normal and optimal production of poultry.
PROBIOTICS: Probiotics contain live microorganisms. Good microorganisms prevail over harmful microorganisms in gastrointestinal tracts of live animals. This ratio can, however, change. Consequently, diarrhoea, poor growth and eventually death can occur. Probiotics inhibit the growth of pathogenic microorganisms by producing organic acids and bacteriocins, by preventing them to attach to intestinal wall, by neutralizing their toxins and encouraging their immune system. They use their own enzymes to improve the digestibility of food. Probiotics enable a better decomposition of a range of vegetable fibres and increase the level of decomposition. Probiotics help to increase the number of favorable microorganisms in the gastrointestinal tract and maintain a favourable intestinal flora.
PREBIOTICS: Prebiotics are the indigestible part of feed which have a positive influence on animals by stimulating growth and reproduction of favourable microorganisms in the gastrointestinal tract. They slow down the development of harmful and pathogenic microorganisms and have a positive influence on animal’s health and breeding results. The chemical status of prebiotics is oligosaccharides, which stimulate the growth of Bifidobacteria.
AROMAS: Aromas, ethereal oils and herbs do not have any nutritional value, but their use improves the appetite and the absorption of nutrients.
FATTY ACIDS: Fatty acids used in feed for laying hens increases the amount of fatty acids in eggs. Omega-3 fatty acids are added to feed. People cannot produce omega-3 fatty acids themselves. They have to be acquired with food. Their intake decreases the possibility of cardiovascular diseases. By adding omega-3 fatty acids to chicken feed we can increase their content in eggs by 3.5 times.
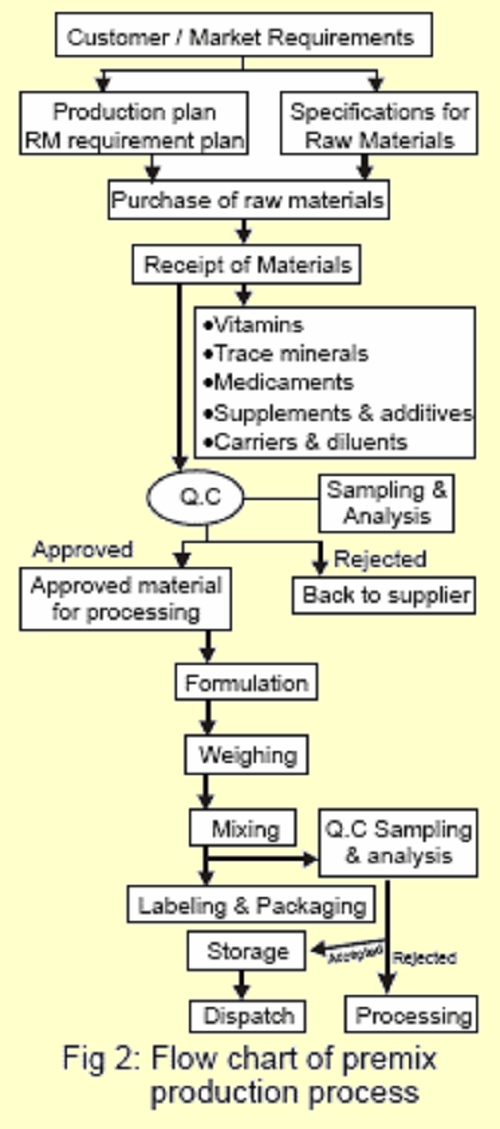
Premix manufacturing process comprises
- Raw Materials
(a) Selection & Specifications
(b) Purchase
(c) Receipt & Storage
(d) Sampling & Analysis
(e) Processing - Formulation
- Weighing
- Mixing
- Packaging
- Labeling
- Storage of Finished Premix
1: RAW MATERIALS
(a) Selection & Specifications
Vitamins and trace minerals are available in different forms and their bioavailability varies between sources. Amongst vitamins the stability forms important criteria whilst bioavailability, potency and reactivity of trace minerals aid in their selection process. The form of ingredient selected must also be easily available, of economic interest and also impart acceptable physical attributes to the premix. In order to maintain the stability of vitamins throughout their shelf life it is recommended to procure more stable derivatives like coated forms, that are not destroyed even when mixed with trace minerals. The spray-dried form of vitamins improves the flowability of the premix. The specifications for all raw materials should be based on recommendations applicable for particular animal’s feed as mentioned by AAFCO, AOAC, AFMA, I.P, U.S.P., etc
(b) Purchase of Raw Materials
Raw materials must be procured from approved vendors and should conform to the specifications laid down by the nutritionist. No material should be received without a certificate of analysis. Purchases should be done periodically taking care that sufficient inventory is maintained at all times. A purchase plan is desirable in accordance with production requirement.
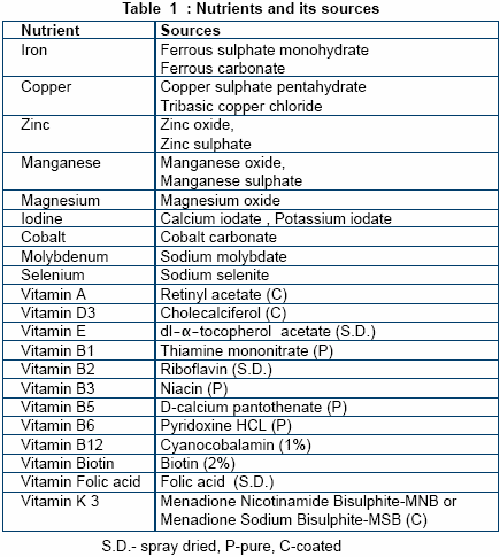
(c) Receipt & Storage of Raw Materials
The receiver should have enough information from the quality assurance program to be able to recognize the quality of product. Sacked ingredients should be checked for identification and condition. A reference number should be allotted for each raw material received into the premises. The sacked ingredients must then bear this reference number. Large consignments are to be weighed at weigh bridge whereas small ones by using electronic balance. The complete details of the raw material along with its reference number must be entered into the stock records.
The raw materials should be logged in after segregation of drugs and other nutrients. Extra care must be taken for labeling. Bags or containers must be stored in a dry location on pallets assigned to them taking care of sufficient space between the pallets for comfort loading and unloading. To prevent development of stacking resistance not more than 10 bags are to be stored on one pallet. Meanwhile stacked stocks should be rotated to minimize lump formation, product degradation, and insect infestation. The storage area must have sufficient protection from rodents and insects. It should be well ventilated, sanitized and away from direct sunlight. Depending on the stability of raw material they must be stored in environment of controlled temperature and humidity.
(d) Sampling & Analysis
Sampling of raw materials is performed following a quality assurance programme. To obtain a representative sample, sampling should be done from bottom, center and top layer of the bag using a sample probe. When large consignments of raw materials are received, it is advisable to mix the raw material in mixer and then analyze each mixed batch to make an accurate assessment. Instruments like H.P.L.C, flame photometer and spectrophotometer are used for analysis of raw materials to obtain accurate results.
(e) Processing
Processing seeks to modify the physical properties of raw materials to meet the specifications of premix. Processing basically includes:
- Sieving
- Milling
Sieving is a primary process of removing foreign materials from raw materials as well as separating coarse ingredients. The operation can be carried out in equipments like vibratory or mechanical sifters. Care must be taken that the sifter is cleaned well before and after use to prevent any sort of contamination. A multimill can be used to reduce particle size to the desired screen analysis. The sieved and milled material is then bagged, weighed, labeled and transferred to the warehouse area for storage.
2. FORMULATION
This is an important and critical step in manufacturing a premix. Qualified personnel possessing knowledge and expertise regarding micro ingredients and powder technology should formulate a premix. The formulator has to consider source of ingredients based on their physical, chemical characteristics, bioavailability, their interactions when mixed, handling characteristics, and economic implications on the final product before any final conclusion is arrived at.
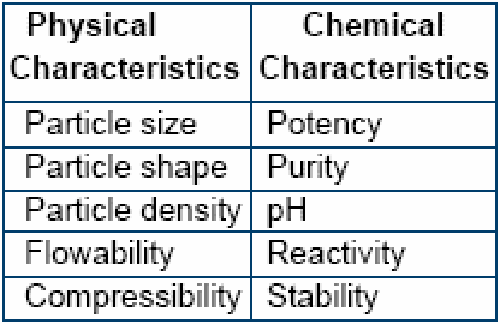
PHYSICAL CHARACTERISTICS
Particles of uniform size, density and that of spherical shape blend well to form a homogenous mixture. The chance of segregation is thereby minimized. For example trace minerals available in the market are invariably found to be coarse in nature whereas the vitamins are normally fine powders. Achieving a homogenous mixing of these two ingredients would be difficult (sand and pebble effect). The flow ability of the ingredients plays a vital role while handling the powder i.e. before and after mixing. Poor flowability results in bridiging, caking and product loss in the transfer system. Conversely too fluid a product may cause flushing.
CHEMICAL CHARACTERISTICS
Potency of vitamins, trace minerals and medicaments need to be considered whilst formulating high quality premixes. Selection of carrier and its percentage is important for formulating a quality premix. It is generally preferable to leave sufficient space for a carrier in order to minimize any sort of interactions between the active ingredients. The carrier should serve the functions as depicted below:
- It should neutralize the electrostatic charges present in certain ingredients.
- Chemically inert
- Primarily have the density, particle shape and particle size compatible with other micro ingredients so as to prevent any demixing in the premix.
- Water sequester from other raw materials thereby reducing water activity and improve stability of the premix.
- Impart good flowability. Types of carrier widely used in the formulation of premix:
- Organic carriers
- Inorganic carriers
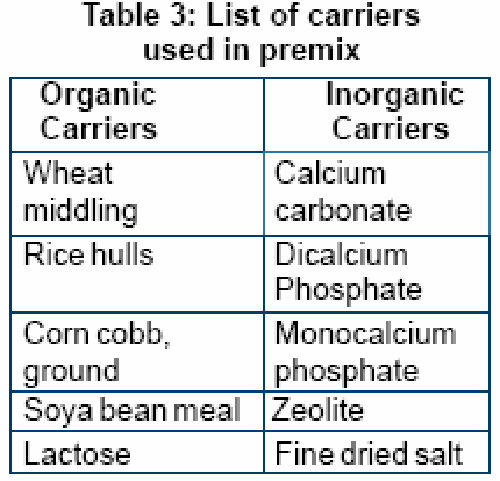
Better premixes can often be prepared by employing a blend of predetermined ratio of several diluents rather than with just one. The organic carrier absorbs moisture while inorganic carrier contributes towards density of premix.
The formulator has to consider all the above – mentioned parameters whilst preparing the batch control or manufacturing record. The batch manufacturing record serves as a link between the formulator and actual production. While preparing the batch sheet the formulator has to give importance for the following details:
- Nutrient requirements
- Selection of ingredients
- Potency of ingredient
- Process loss
- Level of free flowing agents
- Level of antioxidant
- Percentage of carrier
- Packing & packaging material
- Inventory of materials
The manufacturing of a premix should follow the batch control sheet under the supervision of trained personnel. The batch sheet should comprise following details:
- Name of the premix
- Code of premix
- Production date
- Batch no. of premix
- Batch size
- List of ingredients to be mixed
- Batch no. of ingredients to be mixed
- Mixing order of ingredients
- Actual quantity of ingredients to be taken
- Mixer name and mixing time
- Instructions regarding packing and mixing
- Provision for signatures
3. WEIGHING
Weighing is an important point in manufacturing of a premix. The accuracy of the weighing balance enables precise weighing. The accuracy decreases with increasing size of the scale. As a general rule, a scale is accurate to no more than 0.1% of its total capacity. There is large variation in doses of different micro ingredients added in the premix. So weighing balances need to be sized according to their use as depicted left.
4. MIXING
The mixing process is the heart of any premix-manufacturing unit. In a premix the proportion of ingredients vary considerably; hence in order to obtain a homogenous blend the mixing operation should be divided into two steps, A) Micro mixing and B) Macro mixing
5. PACKAGING
The primary purpose of packaging for premix is to maintain the stability of micronutrients and to protect the integrity of the premix. Improperly packaged premixes experience considerable loss in the potency of various sensitive ingredients. Selection or designing of packaging material should be according to the local climatic conditions. It should bear following properties:
- Provides barrier against light, moisture, oxygen
- No chemical interactions with the premix
- Provides good printing surface
- Sturdy enough to withstand the transport pressure.
The different types of packaging materials that could be use are glass containers, aluminium foil, paper and plastics. Ideally, aluminium foil lined multilayered paper bags provides an excellent barrier against light, moisture, oxygen, odour and flavour. Hence for very sensitive ingredients and where cost is not a constraint, aluminium foil package is the material of choice.
6. LABELING
Labeling of premix serves two purposes:
- Provides complete information about the premix
- Gives an identity to the premix and helps in differentiating from other premixes. The premixes for different segments like layer, broiler, breeder and dairy should bear labels of different colour. This prevents any confusion and mix-ups between the premixes.
A premix label should have following information:
- Name of the premix
- Composition
- Dosage of premix
- Net weight of premix contained in the package (in kg)
- Regulatory/Statuatory statements.
- Date of manufacture in month/year
- Date of expiry in month/year
- Batch number
- Storage conditions
- Directions for use
- Name and address of the manufacturer with logo
- Disclaimer note if any
Careful attention must be paid while preparing label since the customer follows the instructions given on the label. Any mistake made will be carried on to the feed and ultimately affect the performance of the birds.
7. STORAGE
- The quality of premix is also affected by the storage conditions in the premises until it is transported through distribution channels. The following steps are recommended during warehouse storage:
- The temperature and humidity of warehouse should be controlled below critical levels.
- Keep the area clean, well lit and ventilated with fresh air.
- Store the premix on the pallet meant for it taking care not to store more than 10 bags on each pallet.
- Design the storage areas to facilitate the FIFO (First in First Out) policy, with bags stored in consecutive order so that oldest can be withdrawn first.
- Make separate provision for storing sale return or expired premix
- Keep floors, walls and walkways clean, dry and free of any obstructions.
- Place sinks and bathrooms away from premix storage area.
- Keep the area free from pests and rodents
- No bag should be stored without any label. When stored under such conditions the consumer is guaranteed of its label claim.
METHODS OF QUALITY CONTROL
- For raw materials
- For production process
- For finished premix
- The raw materials must be analysed first and only then incorporated in the premix once it meets all the specifications. The formulator must be strict while considering the nutrient percentage in the raw material. Proper segregation must be done for the approved and rejected materials. Care should be exercised while actually taking the material for production. The raw material must be sieved to omit any foreign and oversized material, if necessary process before use.
- The Premix production process ensures the precise weighing & inclusion of each required micro-ingredient in the premix. This method must be fool proof and verifiable through a system of physical (weight) and book (record) checks.
- Cross contamination should be eliminated while manufacturing quality premix. It becomes a matter of concern when drugs get carried over from one premix to another that is meant for another segment and where the contaminated drug is toxic to other species.
Reference
- A.J. Leslie. Quality control in feed milling, procedures for an effective program, ASA.
- D. Armstrong and K. Behnke. Premixing MF-2056, Dept. of Grain Science and Industry.
- D. Monsalve. Key points in mixing and delivery of micro ingredients in Feed Formulations. Micro ingredient Premixing, Keeping Current. BASF Corporation. Quality assurance as applied to micronutrient fortification. Guidelines for technicians, supervisors and workers concerned with Nutition. Eds P. Nestel, R. Nalubola, and E.Mayfield.(ILSI)
- P. Raven & G. Walker. Food and Agricultural Organization of United Nations, 1980.
| The content of the articles are accurate and true to the best of the author’s knowledge. It is not meant to substitute for diagnosis, prognosis, treatment, prescription, or formal and individualized advice from a veterinary medical professional. Animals exhibiting signs and symptoms of distress should be seen by a veterinarian immediately. |






Be the first to comment