Nutrition
Antoine Lavoisier (1743-1794) is acknowledged as the Founder of the Science of Nutrition. The series of various chemical reactions & physiological processes which transform food into body tissues and activities. It involves the ingestion, digestion & absorption of the various nutrients, their transport to all body cells and the removal of unusable elements and waste products of metabolism. So, animal nutrition is the science of nourishment of animals.
Nutrients
Nutrients are chemical substances required by the body to sustain basic functions and are optimally obtained by eating a balanced diet. There are six major classes of nutrients essential for health: carbohydrates, lipids, proteins, vitamins, minerals, and water. Carbohydrates, lipids, and proteins are considered macronutrients and serve as a source of energy. Water is required in large amounts but does not yield energy.
Minerals and Vitamins are considered micronutrients and play essential roles in metabolism. Minerals are inorganic micronutrients. Minerals can classify as macro-minerals or micro-minerals. Macrominerals are required in amounts greater than 100 mg per day and include calcium, phosphorous, magnesium, sodium, potassium, and chloride. Sodium, potassium, and chloride are also electrolytes. Micro-minerals are those nutrients required in amounts less than 100 mg per day and include iron, copper, zinc, selenium, and iodine. Vitamins are organic micronutrients classified as either water-soluble or fat-soluble. The essential water-soluble vitamins include vitamins B1, B2, B3, B5, B6, B7, B9, B12, and C. The essential fat-soluble vitamins include Vitamin A, E, D, and K.
Nutrient Interactions
Nutrient–nutrient interaction in the process of digestion and absorption of nutrients. The requirements for micronutrients are high in newborn animals because of the need to form new tissues in growth.
Mechanisms of Interactions
The term “Interaction” is defined by O’Dell (1997) as “Interrelationships among mineral elements as revealed by physiological or biochemical consequences”.
There are two major classes of interactions, positive or synergistic, and negative or antagonistic. Georgievskii (1982) described 16 synergistic interactions among 15 of the required mineral elements.
Georgievskii (1982) listed 26 antagonistic interactions among 15 of the required elements.
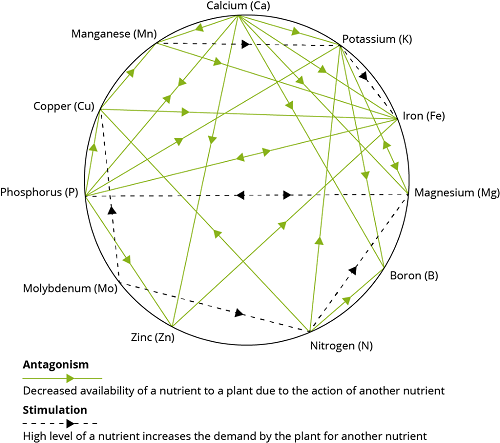
With the advancement of knowledge and discovery of new element, it has become essential to understand the interrelationship between minerals and vitamins because many of biological processes depend upon the availability of both the micronutrients.
One popular theory of mineral interactions based on chemical and physical properties of the elements was proposed by Hill and Matrone (1970). Ions with similar ionic size or electron configurations of their outer orbitals are likely to interact competitively. Most of the recognized essential trace elements are in the first transition series in the Periodic Table and have unfilled 3d orbitals.
Some important Mineral- Mineral, Mineral– Vitamin interaction are
- Copper-Molybdenum-Sulfur Interaction
- Copper-Iron-Zinc Interaction
- Selenium- Iodine- Sulfur
- Manganese – Iron Interaction
- Zinc /Manganese –Phytate Interaction
- Calcium, Phosphorus And Vitamin D Interaction
- Calcium, Manganese And Vitamin K Interaction
- Magnesium –Calcium- Phosphorus Interaction
- Selenium And Vitamin E Interaction
- Cobalt – Vitamin B12 Interaction
- Folate And Vitamin B12 Interaction
- Zinc – Vitamin A Interaction
- Vitamin A And Iodine Interaction
- Vitamin A, E And K Interaction
- Vitamin C With Minerals Interaction
- Sulphur- Thiamine- Biotin Interaction
1. Copper-Molybdenum-Sulfur Interaction
One interaction of practical importance in ruminant nutrition, recognized since the 1950s, is the copper-molybdenum-sulfur (Cu-Mo-S) interrelationship (Dick, 1953). In the reducing environment of the rumen, dietary sulfur is reduced to sulfide, which then interacts with molybdenum to form thiomolybdates (Mason, 1986). Thiomolybdates have been shown to affect copper metabolism in two ways. In the gastrointestinal tract, some thiomolybdates have been shown to bind copper preventing its absorption.
Thiomolybdates that are absorbed have also been shown to cause systemic effects on copper metabolism including:
- Increased biliary excretion of copper from liver stores
- Reduced transport of available copper for biochemical processes and
- Removal of copper from metalloenzymes (Spears, 2003).
The formation of thiomolybdates appears to depend on total dietary sulfur concentration. Spears (2003) found data to show that at low ruminal sulfide concentrations, molybdenum may have little effect on copper availability, whereas at higher ruminal sulfide concentrations copper availability was significantly decreased.
The teartness or peat scour characterized by unthriftiness and scouring is due to high molybdenum content in the pasture i.e. 20 to 100 mg/kg DM as compare to 0.5 to 3.0 mg/kg DM in normal pasture and thus teart was originally regarded as molybdenosis. However feeding with Cu sulphate controlled the scouring and hence a Mo-Cu relationship was established. It is now known that Mo is complex and exert a limiting effect on Cu retention in the animal in the presence of sulphate. Sulphide is formed by ruminal micro-organism from dietry sulphate or organic sulphur compounds. The sulphide then react with Mo to form thiomolybdate which is turn combined with copper to form insoluble copper thio-molybdate (Cu-Mo-S4) thereby limiting the absorption of dietry Cu. It is considered like that if thio-moylbdate is formed in excess it may be absorbed from the digestive tract and exert a systemic effect on Cu metabolism in the animal. In some places, deficiency has been noticed even through the forage containing sufficient amount of copper due to excess of Mo or inorganic sulphate in the fodder. This type of deficiency is known as conditional or induced deficiency.
Copper Antagonist– Molybdenum and Zinc. High level of Zn and Cd depress copper absorption. Silver, calcium carbonate, ferrous sulphate and calcium phytate interfere with copper absorption.
Arthington (2003) described other sources of sulfur that could also contribute to both the Cu-Mo-S and Cu-S interactions. Sources included fertilizers, high sulfur water, and sulfur containing supplements. Forages fertilized with ammonium sulfate may have a much higher concentration of sulfur compared with forages fertilized with ammonium nitrate. Arthington (2003) suggested that sulfate in water may be more available to interact with Mo and Cu compared with sulfur from dietary sources, and is a greater management challenge for livestock producers compared with sulfate fertilizers.
2. Copper-Iron-Zinc Interaction
There is a synergistic effect of copper on iron as the copper containing enzyme ceruloplasmin catalyzes the oxidation of Fe+2 to Fe+3. Which is necessary for binding to transferrin to take place for transport of iron in plasma. Mobilization of iron from storage sites in mucosa and liver does not occur at a normal rate during copper deficiency with resulting anemia observed as a consequence.
Ruminants consuming forage based diets can be exposed to high levels of iron. Grazing animals can consume high levels of iron as a consequence of soil consumption during grazing and through consumption of soil contaminated forages. During winter, soil ingestion can exceed 10% of the dry matter intake of grazing sheep and cattle. This level of soil ingestion markedly reduced copper absorption. High dietary iron has been shown to reduce copper status in cattle and sheep. It is believed that sulfide in the rumen combines with iron to form ferrous sulfide complexes that dissociate in the low pH abomasum where the sulfide then forms insoluble complexes with copper (Gengelbach et al., 1994).
Copper is affected negatively by excess dietary zinc and iron, as well as the copper molybdenum- sulfur interaction. High dietary concentrations of zinc will induce synthesis of metallothinein protein in the intestinal cells to protect the animal against zinc toxicosis. Copper has a higher affinity for the protein than zinc and it was speculated originally to cause a secondary copper deficiency. There is a mutual inhibitory interaction between iron and zinc. Part of this effect may take the form of excess zinc inhibiting absorption of copper with a resulting copper deficiency causing the iron deficiency anemia. There is also a direct inhibitory effect of zinc on cellular uptake of iron.
3. Selenium- Iodine- Sulfur Interaction
The major mineral interactions with selenium of practical importance involve iodine and sulfur. Selenium can replace sulfur in amino acids such as cysteine and methionine which are then incorporated into body proteins. Selenium may be absorbed via the pathways for these amino acids. Selenium is also an integral part of the enzyme thyroxin 5′-deiodinase, which converts thyroxin (T4) to triiodothyronine (T3), the more active form of the hormone (Arthur, 1992). Selenium deficiency blocks the thyroid feedback-control system resulting in larger goiters than if iodine alone were deficient. Heifers grazing marginally selenium-deficient pastures supplemented with intra-ruminal selenium pellets had decreased T4 and increased T3 concentrations. At excessive pharmacological concentrations copper and zinc, iron, cadmium, mercury, silver, and arsenic have been reported to interfere with utilization of selenium.
4. Manganese – Iron Interaction
Non-heme iron shares a mutual inhibition pathway with manganese. In addition, manganese competes with iron for binding sites on the plasma iron carrier protein transferrin, inhibiting mobilization and transport of iron (Davidsson et al., 1989).
5. Zinc /Manganese -Phytate Interaction
In the 1950s, the association between zinc deficiency and parakeratosis in swine was established (Tuker and Salmon, 1955). Excess dietary phosphorus, and to a lesser extent, calcium have an antagonistic effect on absorption of manganese in swine, poultry and dairy calves (Henry, 1995). Dietary phytate has also been reported to inhibit absorption of manganese (Halpin and Baker, 1986). Of all the required elements, zinc has been shown to form the most stable complexes with phytate at a physiological pH of 7.0. At pH 4.0 in the media, the inhibitory potency of zinc and iron on phytase activity was decreased. Although high dietary concentrations of zinc affect copper absorption, the reverse interaction does not occur. Zinc (Hahn and Evans, 1975) and phytate (Chen et al., 1973) have been reported to decrease absorption of chromium.
6. Calcium, Phosphorus and Vitamin D Interaction
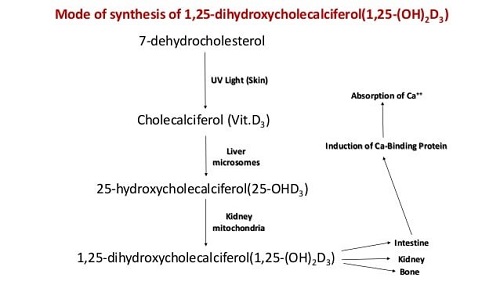
The definite relationship of calcium and phosphorus in the formation of bones and teeth and as the major structural elements of the skeletal tissue. The large amount of Ca and P in bone can be liberated by resorption which takes place particularly during lactation and egg production, although the exchange of Ca and P between bone and soft tissue is always a continuous process. The resorption of Ca is controlled by the action of parathyroid gland. If the animals are fed on low calcium diet, the parathyroid gland is stimulated and the hormone produced causes resorption of bone librated Ca to meet the requirement of the animal. Since Ca is combined with P in bone the P is also librated and excreted by the animal in urine. The parathyroid hormone also plays an important role in regulating the amount of Ca absorbed from the intestine by influencing the production of 1, 25dihydroxy cholecalciferol {1-25(OH)2 D3} a derivative of vitamin D which is concerned with the formation of calcium binding protein known as calmodulin and osteopontin. Vitamin D provides acidic medium in the intestine causing more Ca absorption. Vitamin D promotes absorption through the formation of calcium binding protein (calmodulin and osteopontin). A great excess of either Ca or P interfere the absorption of others. The ratio between Ca and P is more important in non-ruminant then ruminant.
7. Calcium, Manganese And Vitamin K
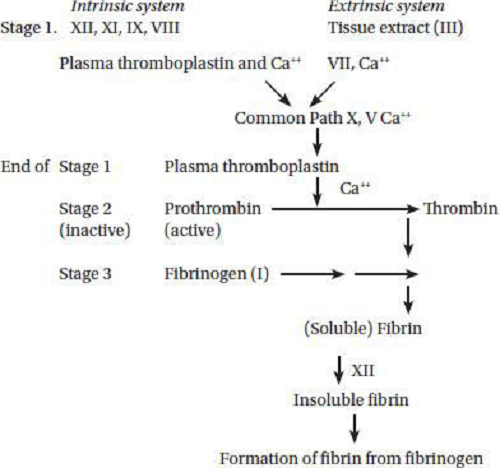
Vitamin K is necessary for the synthesis of prothrombin in the liver which is important intermediate of the blood clotting process and also for the synthesis of factors plasma thromboplastin and tissue thromboplastin involved in the conversion of prothrombin to thrombin. Vitamin K is required for synthesis of prothrombin (factor II) in liver. Prothrombin (factor II) (inactive precursor) is converted to thrombin by carboxylation of glutamic acid residue in the presence of vitamin K dependent carboxylase enzyme. The prothrombin must bind Ca ions before it can be activated. If the supply of vitamin K is inadequate, then the prothrombin molecule is deficient in carboxyglutamic acid, a specific amino acid responsible for calcium binding. Proteins containing carboxyglutamic acid, dependent on vitamin for their formation, are also present in bone, kidney and other tissues. Thrombin acts as an enzyme which converts protein fibrinogen in blood plasma into fibrin, the insoluble fibrous protein that holds blood clots together. The inactive vitamin K dependent zymogens are converted into calcium binding proteins which activate them. Glyosyl transferees, a manganese containing enzyme is essential for prothrombin to thrombin with the help of Vitamin K.
8. Magnesium- Calcium- Phosphorus Interaction
The diet adequate in Mg and other nutrient increasing the Ca and P or both results in symptoms of Mg deficiency which is marked by calcification in soft tissue. There is also evidence of decreased absorption or increased excretion of Mg or both. The Mg may influence the Ca and P utilization but when these two elements are present in liberal amount, the harmful effect not seen. It is reported that injection of Mg sulphate by cattle on low- phosphorus diet result in serious and continuous losses of Ca which are overcome by increasing the P content of the ration.
9. Selenium and Vitamin E Interaction
Selenium and vitamin E interact with each other, and each potentiates the effect of the other (Mervyn, 1985). Organic and inorganic selenium compounds function in preventing certain disease conditions that have, in the past, been associated with vitamin E deficiency (Soetan et al., 2010).
Vitamin E functions in the animal mainly as biological antioxidant at the cellular level in association with selenium-containing enzyme glutathione peroxidase, it protects cells against oxidative damage caused by free radicals. [Free radicals are formed during cellular metabolism and, as they are capable of damaging cell membranes, enzymes and cell nuclear material, they must be converted into less reactive substances if the animal is to survive. This protection is particularly important in preventing oxidation of polyunsaturated fatty acids which function as primary constitute of subcelluar membrane and precursors of prostaglandins. Oxidation of unsaturated fatty acids produces hydro-peroxides, which also damage cell tissues and more lipid free radicals. Per oxidation of lipid can destroy structural integrity of the cells and can cause metabolic derangements causing Vitamin E injury so that prevention of such oxidation is of vital importance in maintaining the health of the living animal].
The animal has two main methods of protecting itself against oxidative damage. Firstly, radicals are scavenged by vitamin E as a first line of defense and secondly, glutathione peroxidase destroys any peroxide formed before they can damage the cell. These two defense mechanisms complement each other. Vitamin E participates in normal tissue respiration possibly by the way of cytochrome reductase system and to protect the lipid structure of mitochondria from oxidative destruction. Vitamin E helps in normal phosphorylation of creatine phosphate from ATP which is high phosphate energy compounds in the body.
Nutritional myopathy or muscular dystrophy- In cattle and calves, in skeletal and heart muscles due to deficiency of vitamin E characterized by muscle weakness and paralysis due to degenerative changes creatine excretion is increased. This is seen in calves when they are turned out on to spring pasture. It is associated with low vitamin E and selenium intakes during the in-wintering period and possibly, the relative high concentration of polyunsaturated fatty acid in young grass lipids, the requirement for vitamin E is increased with increasing concentration of polyunsaturated fatty acid in the diet.
10. Cobalt – Vitamin B12 Interaction:
Cobalt is a component of vitamin B12 approximately 4.5 percent of the molecular weight of B12 (cyanocobalamin) is contributed by elemental cobalt. Cobalt is necessary for the cell growth and development of body as well as for the multiplication of rumen microbes in ruminants. It form an essential part of the enzyme system and play important role in synthesis of vitamin B12 in rumen. But is also involved in synthesis of DNA and metabolism of amino acid. In ruminant B12 is not directly essential but cobalt is essential where as in non ruminant B12 is dietary essential. In ruminant cobalt deficiency because vitamin B12 deficiency. In ruminant cobalt is required by rumen microorganism for synthesis of B12 vitamin deficiency of cobalt leads to insufficient production of B12 to satisfy the animal requirement these symptoms can be overcome by injection of vitamin B12 in the blood but cobalt injection does not improve the condition. It is cheaper two feed cobalt through mouth in the ruminants so that vitamin B12 is synthesized by the microorganism in the rumen. Small amount of B12 also synthesize caecum of monogastric animals but it is not sufficient to meet the requirement of poultry and pig.
11. Folate and Vitamin B12 Interaction
Folate and vitamin B12 play an indispensible role in the methylation cycle, which is the sole methyl group donor in numerous biochemical reactions within the central nervous system and elsewhere, such as neurotransmitter synthesis, hepatocellular detoxification, nerve myelination and DNA stabilization (Gropper et al., 2009). Folacin and vitamin B12 are required for the synthesis of methyl groups and metabolism of the one-carbon unit. Biosynthesis of labile methyl from a formate carbon requires folacin, while B12 plays a role in regulated transfer of the methyl group from tetrahydrofolic acid. Therefore, marked increases in choline requirement have been observed under conditions of folacin and/or vitamin B12 deficiency. Methylation dysfunction may occur in the presence of vitamin deficiencies such as vitamin B12 and folate, as well as abnormalities in their metabolism (Finkelstein, 2007). Vitamin B12 deficiency may induce a secondary folate deficiency by reducing methionine synthase enzyme activity, which activates folate (Gibson, 2005).
Interrelationships with other vitamins (e.g., vitamin B12, vitamin C, and biotin) on pantothenic acid requirements are known (Scott et al., 1982). A fivefold increase in coenzyme A content of liver was found in B12-deficient chicks and rats. The pantothenic acid is involved in ascorbic acid synthesis in plants and animals, with ascorbic acid sparing the pantothenic acid requirement in rats (Barboriak and Krehl, 1957). Also, there have been suggestions of a possible interrelationship between folacin and biotin with pantothenate. Both vitamins were found necessary for pantothenic acid utilization in the rat (Wright and Welch, 1943). The inclusion of biotin in the diet of a pantothenic acid-deficient pig was effective in prolonging the life of the pig, but caused the pantothenic acid deficiency signs to appear in half the time (Colby et al., 1948).
12. Zinc and Vitamin A Interaction
Zinc participates in the absorption, mobilization, transport, and metabolism of micronutrients, including vitamin A, most likely through its involvement in protein synthesis and cellular enzyme functions. Vitamin A and zinc interact during the conversion of retinol to retinal in the retina of the eye during the visual cycle as well as in other tissues such as the liver and testes. The zinc metalloenzyme ADH is required for this oxidative process. The retina is sensitive to zinc nutriture and that zinc deficiency can impair photoreceptor function by down-regulating ADH activity. Rhodopsin, a photosensitive pigment required for night vision, is synthesized from retinaldehyde and a membrane protein moiety, opsin. In zinc deficiency the formation of 13-cis-retinal is reduced, causing a decrease in rhodopsin formation and rod photosensitivity that can lead to poor dark adaptation or night blindness.
13. Vitamin A and Iodine Interaction
Retinoic acid is involved in iodine uptake. Severe vitamin A deficiency decreases the uptake of iodine and impacts thyroid metabolism. Iodine deficiency and vitamin A deficiency leads to a more severe case of primary hypothyroidism compared to iodine deficiency alone.
14. Vitamin A, E and K Interaction
High levels of beta carotene might decrease serum levels of vitamin E. Vitamin A toxicity inhibits the synthesis of vitamin K2 by intestinal bacteria and interferes with hepatic actions of vitamin K. Vitamin A interferes with absorption of vitamin K.
15. Vitamin C with Minerals Interaction:
Dietary ascorbate (vitamin C) exists in the reduced [(i.e. AA (ascorbic acid)] and oxidized [DHAA (dehydro-L-ascorbic acid)] forms. The vitamin acts as a cofactor in a variety of critical metabolic reactions that include the synthesis of collagen, carnitine and catecholamine as well as in peptide amidation and tyrosine metabolism; it is also involved in maintaining metal ions (like iron and copper) in their reduced forms and serves as a scavenger for free radicals.
Most mammals can generate ascorbic acid from D-glucose endogenously. However, humans (and other primates as well as the guinea pig) cannot do so as they lack the enzyme L-gulonolactone oxidase; rather, they obtain the vitamin from dietary sources via intestinal absorption.
16. Sulphur- Thiamine- Biotin Interaction:
The functions of sulfur are as diverse as the proteins of which it is a part. Sulfur is frequently present as highly reactive sulfhydryl (SH) groups or disulfide bonds, maintaining the spatial configuration of elaborate polypeptide chains and providing the site of attachment for prosthetic groups and the binding to substrates that are essential to the activity of many enzymes. Hormones such as insulin and oxytocin contain sulfur, as do the vitamins thiamine and biotin. The conversion between homocysteine and methionine facilitates many reactions involving methylation. Cysteine-rich molecules such as metallothionein play a vital role in protecting animals from excesses of copper, cadmium and zinc, while others influence selenium transport and protect tissues from selenium toxicity. Glutathione may facilitate the uptake of copper by the liver. Glutathione also protects tissues from oxidants by interconverting between the reduced (GSH) and oxidized (GSSG) state, and may thus protect erythrocytes from lead toxicity. Sulfur is present as sulfate (SO4) in the chondroitin SO4 of connective tissue, in the natural anticoagulant heparin and is particularly abundant in the keratin-rich appendages (hoof, horn, hair, feathers, wool fibre and mohair). The rumen fermentation and microbial protein synthesis sulphur is required. (Durand and Komisarczuk, 1988).
Conclusion
In view of the importance of these macro and micro mineral elements in the nutrition of animals and plants and their metabolic inter-relationships which influences other vital factors needed for the survival of living organisms like enzymes, anti-oxidants, vitamins etc, it is important to regularly obtain up-to-date information on the minerals content of water and the variously commonly consumed plant foods used for animal foods and feeds respectively e.g. legumes, cereals, fruits and vegetables. This is because of the effects of chemicals like pesticides, herbicides, fertilizers on the mineral contents of the soils where these plants are cultivated. Genetic, location and environmental factors could also influence the levels of the mineral elements in plants.
References
- Allen J. D. and Gawthome J. W. (1987). Involvement of the solid phase of rumen digesta in the interaction between copper, molybdenum, and sulphur in sheep. Br. J. Nutr 58: 265.
- Arthington J. D. (2003). Copper antagonists in cattle nutrition. Proceedings 14th Annual Florida Ruminant Nutrition Symposium, Gainesville, Florida. P. 48.
- Atrhur J. R. (1992). Interrelationships between selenium deficiency, iodine deficiency, and thyroid hormones. An. J. Clin. Nutr 57: 2355.
- Barboriak J.J. and Krehl W.A. (1957). J. Nutr 63:601.
- Boland M.P., Lonergan P. and Callaghan O. (2001). Effect of nutrition on endocrine parameters, ovarian physiology, and oocyte and embryo development, Theriogenology 55:1323-1340.
- Chen N. S. C., Tsai A. and Dyer. I.A. (1973). Effect of chelating agents of chromium absorption in rats. J. Nutr. 103: 1182.
- Colby R.W., Cunha T.J., Lindley C.E., Cordy D.R. and Ensminger M.E. (1948). J. Am. Vet.Med. Assoc 113: 589.
- Cromwell G.L. (1997). Handbook of copper compounds and applications. pp 177-202.
- Davidsson L., Walczyk T., Zavaleta N. and Hurrell R.F. (2001). Improving iron absorption from a Peruvian school breakfast meal by adding ascorbic acid or Na2EDTA. Am. J. Clin. Nutr 73: 283– 287.
- Dick A. T. (1953). The control of copper storage in the liver of sheep by inorganic sulphate and molybdenum. Aust. Vet. J 29: 233.
- Durand M. and Komisarczuk K. (1988) Influence of major minerals on rumen microbiota. J. Nutri 118: 249–260.
- Eastmond D.A. MacGregor J.T. and Slesinki R.S. (2008). Trivalent Chromium: Assessing the genotoxic risk of the essential trace element and widely used human and animal nutritional supplement. Crit. Rev. Toxicol 38: 173-190.
- Frieden E. (1984). Biochemistry of the essential ultra trace elements. Plenum press, New York.
- Gengelbach G. P., Ward J. D. and Spears J. W. (1994). Effect of dietary copper, iron, and molybdenum on growth and copper status of beef cows and calves. J. Anim. Sci 72: 2722.
- Georgievskii V. I. (1982). General information on minerals. pp. 11-56. Butterworths, London.
- Goff J.P. (1999). Dry cow nutrition and metabolic disease in parturient cows. Proceeding Western Canadian Dairy Seminar Red Deer. Dairy Cattle Reproduction Council (2010). Finding the nutritional balance for a Successful reproduction program. pp 8-10.
- Hahn C. J. and Evans G.W. (1975). Absorption of trace metals in the zinc deficient rat. Am. J. Physiol 228: 1020.
- Halpin K. M. and Baker D. H. (1986). Manganese utilization in the chick: Effects of corn, soybean meal, fish meal, wheat bran and rice bran on tissue uptake of manganese. Poult.Sci 65: 995.
- Hays V.W. and Swenson M.J. (1985). Minerals and Bones. In: Dukes’ Physiology of Domestic Animals, Tenth Edition pp. 449-466.
- Henry P.R. and Ammerman C.B. (1995). Selenium bioavailability. In: C. B. Ammerman, D.H. Baker and A. J. Lewis (Eds.) Bioavailability of nutrients for animal, amino acids, minerals, and vitamins. pp. 303-336. Academic Press, San Diego.
- Hess B.W., Moss G.E. and Rule D.C. (2008). A decade of developments in the area of fat supplementation research with beef cattle and sheep. J. Ani. Sci 86: 188-204.
- Hill C. H. and Matrone G. (1970). Chemical parameters in the study of in vivo and in vitro interactions of transition elements. Fed. Proc 29: 1474.
- Huber A.M. and Gershoff S.N. ( 1975). Effects of zinc deficiency on the oxidation of retinol and ethanol in rats. J. Nutr 105: 1486–90.
- Kappel L.C. and Zidenberg S. (1999). Manganese: Present Knowledge in nutrition, In: Brown ML (Ed.), International Life Sciences Institute Nutrition Foundation, Washington, pp 308.
- Lonnerdal B. (2003). Interaction between Micronutrients: Synergies and Antagonisms, In: Micronutrient Deficiencies during the Weaning Period and First Years of Life. J M Petti for and S Zlotkin. Nestle Nutrition Workshop Series Paediatric Program, 54: 8-12.
- Malhotra V.K. (1998). Biochemistry for Students. Tenth Edition. Jaypee Brothers Medical Publishers (P) Ltd, New Delhi, India.
- Mason J. (1986). Thiomolybdates: Mediators of molybdenum toxicity enzyme inhibitors Toxicol. 42: 99.
- Mejia L.A.(1986). Vitamin A–nutrient interrelationships. In: Bauernfeind J.C, ed. Nutrition basics and applied science—a series of monographs. New York: Academic Press Inc, 89-100
- Terhune M.W. and Sandstead H. H.(1972). Decreased RNA polymerase activity in mammalian zinc deficiency. Sci 177: 68- 9.
- Merck V.M. (1986). The Merck Veterinary Manual. Sixth Edition. A handbook of diagnosis, therapy and disease prevention and control for the veterinarian. Published by Merck and Co., Inc., Rahway, New Jersey, USA.
- Murray R.K., Granner D.K., Mayes P.A. and Rodwell V.W. (2000). Harper’s Biochemistry, 25th Edition, McGraw-Hill, Health Profession Division, USA.
- Nix K.J., Roberts S. and Wiltbank J.N. (1981). Using short-term calf removal and flushing to improve pregnancy rate. Beef Cattle Research in Texas. Texas Agricultural Experiment Station.
- O’Dell, B. L. (1997). Mineral-ion interaction as assessed by bioavailability and ion channel function. In: B.L. O’Dell and R. A. Sunde (Eds.) Handbook of nutritionally essential mineral elements. pp. 641-659. Marcel Dekker, Inc., New York.
- Scott M.L., Nesheim M.C. and Young R.J. (1982). Nutrition of the Chicken.
- Smith J.E., Brown E.D. and Smith J.C. (1974). The effect of zinc deficiency on the metabolism of retinol binding protein in the rat. J. Lab. Clin. Med 84: 692–7.
- Spears J. W. (2003). Trace mineral bioavailability in ruminants. J. Nutr. 133:150-163.
- Suharno D., West C.E., MuhilalKaryadi D. and Hautvast J.G.A. (1993). Supplementation with vitamin A and iron for nutritional anaemias in pregnant women in West Java, Indonesia. Lancet, 342:1325–1328.
- Sundaresan P.R., Cope F.O. and Smith J.C. (1977). Influence of zinc deficiency on retinal reductase and oxidase activities in rat liver and testes. J. Nutr 107: 2189–97.
- Suttle N. F. (1975). An effect of soil ingestion on the utilization of dietary copper by sheep. J.Agric. Sc Cam 84: 249.
- Wald G. (1950). The interconversion of the retinenes and vitamin A in vitro. Biochem Biophys Acta 4: 215–28.
- Wennberg A. (1994). Neurotoxic effects of selected metals. Scand. J. Work. Environ. Health 20: 65-71.





Be the first to comment